LyonisideCAS# 34425-25-7 |
- Nudiposide
Catalog No.:BCN7437
CAS No.:62058-46-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34425-25-7 | SDF | Download SDF |
| PubChem ID | 14521039 | Appearance | Powder |
| Formula | C27H36O12 | M.Wt | 552.6 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
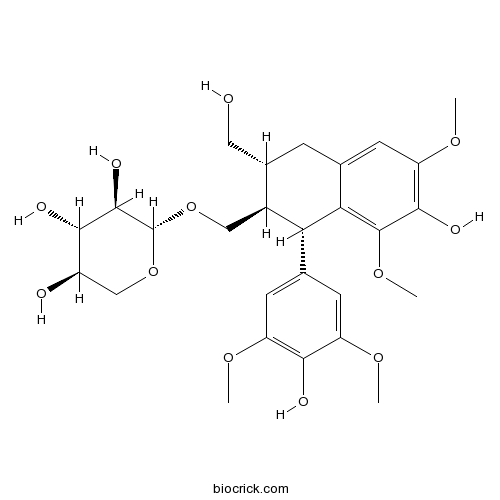
| Chemical Name | (2R,3R,4S,5R)-2-[[(1S,2R,3R)-7-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-6,8-dimethoxy-1,2,3,4-tetrahydronaphthalen-2-yl]methoxy]oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C(C(CC3=CC(=C(C(=C23)OC)O)OC)CO)COC4C(C(C(CO4)O)O)O | ||
| Standard InChIKey | GWDZRGQRNHELQM-VEKSOEEBSA-N | ||
| Standard InChI | InChI=1S/C27H36O12/c1-34-17-7-13(8-18(35-2)23(17)31)20-15(10-38-27-25(33)22(30)16(29)11-39-27)14(9-28)5-12-6-19(36-3)24(32)26(37-4)21(12)20/h6-8,14-16,20,22,25,27-33H,5,9-11H2,1-4H3/t14-,15-,16+,20+,22-,25+,27+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lyoniside and saracoside are cytotoxic to promastigotes and intracellular amastigotes, they demonstrate strong anti-leishmanial efficacies in BALB/c mice model of leishmaniasis, suggests that these two compounds potential anti-leishmanial candidates. 2. The synergistic action of lyoniside and triterpene acids was demonstrated in inhibitory effect exerted on germination and growth of Pinus sylvestris . |
| Targets | Antifection |

Lyoniside Dilution Calculator

Lyoniside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8096 mL | 9.0481 mL | 18.0963 mL | 36.1925 mL | 45.2407 mL |
| 5 mM | 0.3619 mL | 1.8096 mL | 3.6193 mL | 7.2385 mL | 9.0481 mL |
| 10 mM | 0.181 mL | 0.9048 mL | 1.8096 mL | 3.6193 mL | 4.5241 mL |
| 50 mM | 0.0362 mL | 0.181 mL | 0.3619 mL | 0.7239 mL | 0.9048 mL |
| 100 mM | 0.0181 mL | 0.0905 mL | 0.181 mL | 0.3619 mL | 0.4524 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3-dihydrosciadopitysin
Catalog No.:BCN4034
CAS No.:34421-19-7
- Lathyrol
Catalog No.:BCN4963
CAS No.:34420-19-4
- 8-Chloroadenosine
Catalog No.:BCC7935
CAS No.:34408-14-5
- Boc-Lys(Z)-OSu
Catalog No.:BCC3418
CAS No.:34404-36-9
- H-D-Lys(Z)-OH
Catalog No.:BCC2678
CAS No.:34404-32-5
- Boc-D-Glu-OBzl
Catalog No.:BCC3394
CAS No.:34404-30-3
- Betamipron
Catalog No.:BCC8876
CAS No.:3440-28-6
- H-D-Pro-OH
Catalog No.:BCC3023
CAS No.:344-25-2
- N-Demethyl-alpha-obscurine
Catalog No.:BCN7362
CAS No.:34399-44-5
- Isovallesiachotamine
Catalog No.:BCN3549
CAS No.:34384-71-9
- Acebutolol HCl
Catalog No.:BCC4322
CAS No.:34381-68-5
- CP-673451
Catalog No.:BCC4981
CAS No.:343787-29-1
- Ikshusterol
Catalog No.:BCN5278
CAS No.:34427-61-7
- Ligularine
Catalog No.:BCN2090
CAS No.:34429-54-4
- Tirandamycin A
Catalog No.:BCN1861
CAS No.:34429-70-4
- Prudomestin
Catalog No.:BCN5279
CAS No.:3443-28-5
- Alpha-Onocerin diacetate
Catalog No.:BCN6700
CAS No.:34434-99-6
- Amycomycin
Catalog No.:BCN1824
CAS No.:344362-08-9
- Nortrachelogenin
Catalog No.:BCN5280
CAS No.:34444-37-6
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- 17-ODYA
Catalog No.:BCC6717
CAS No.:34450-18-5
- Leukadherin 1
Catalog No.:BCC6332
CAS No.:344897-95-6
- Araloside X
Catalog No.:BCN2467
CAS No.:344911-90-6
The lignan glycosides lyoniside and saracoside poison the unusual type IB topoisomerase of Leishmania donovani and kill the parasite both in vitro and in vivo.[Pubmed:24134912]
Biochem Pharmacol. 2013 Dec 15;86(12):1673-87.
Lignans are diphenyl propanoids with vast range of biological activities. The present study provides an important insight into the anti-leishmanial activities of two lignan glycosides, viz. Lyoniside and saracoside. These compounds inhibit catalytic activities of topoisomerase IB (LdTopIB) of Leishmania donovani in non-competitive manner and stabilize the LdTopIB mediated cleavage complex formation both in vitro and in Leishmania promastigotes and subsequently inhibit the religation of cleaved strand. These two compounds not only poison LdTopIB but also can interact with the free enzyme LdTopIB. We have also shown that Lyoniside and saracoside are cytotoxic to promastigotes and intracellular amastigotes. The protein-DNA complex formation leads to double strand breaks in DNA which ultimately triggers apoptosis-like cell death in the parasite. Along with their cytotoxicity towards sodium antimony gluconate (SAG) sensitive AG83 strain, their ability to kill SAG resistant GE1 strain makes these two compounds potential anti-leishmanial candidates. Not only they effectively kill L. donovani amastigotes inside macrophages in vitro, Lyoniside and saracoside demonstrated strong anti-leishmanial efficacies in BALB/c mice model of leishmaniasis. Treatment with these lignan glycosides produce nitric oxide and reactive oxygen species which result in almost complete clearance of the liver and splenic parasite burden. These compounds do not inhibit human topoisomerase IB upto 200muM concentrations and had poor cytotoxic effect on uninfected cultured murine peritoneal macrophages upto 100muM concentrations. Taken together it can be concluded that these compounds can be developed into excellent therapeutic agent against deadly disease leishmaniasis.


