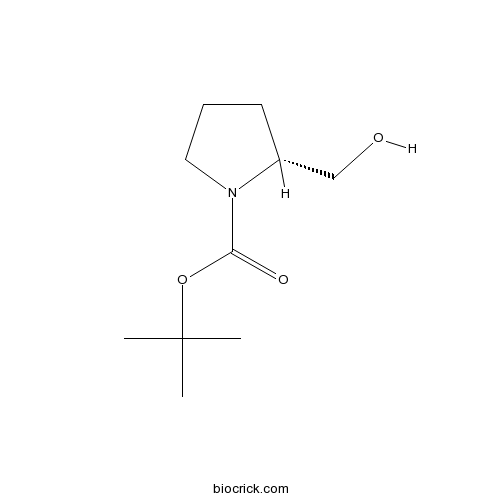
Boc-D-ProlinolCAS# 83435-58-9 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83435-58-9 | SDF | Download SDF |
| PubChem ID | 688279 | Appearance | Powder |
| Formula | C10H19NO3 | M.Wt | 201.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | tert-butyl (2R)-2-(hydroxymethyl)pyrrolidine-1-carboxylate | ||
| SMILES | CC(C)(C)OC(=O)N1CCCC1CO | ||
| Standard InChIKey | BFFLLBPMZCIGRM-MRVPVSSYSA-N | ||
| Standard InChI | InChI=1S/C10H19NO3/c1-10(2,3)14-9(13)11-6-4-5-8(11)7-12/h8,12H,4-7H2,1-3H3/t8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-D-Prolinol Dilution Calculator

Boc-D-Prolinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9677 mL | 24.8385 mL | 49.6771 mL | 99.3542 mL | 124.1927 mL |
| 5 mM | 0.9935 mL | 4.9677 mL | 9.9354 mL | 19.8708 mL | 24.8385 mL |
| 10 mM | 0.4968 mL | 2.4839 mL | 4.9677 mL | 9.9354 mL | 12.4193 mL |
| 50 mM | 0.0994 mL | 0.4968 mL | 0.9935 mL | 1.9871 mL | 2.4839 mL |
| 100 mM | 0.0497 mL | 0.2484 mL | 0.4968 mL | 0.9935 mL | 1.2419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-D-Prolinol
- ICI 154,129
Catalog No.:BCC5677
CAS No.:83420-94-4
- Phenformin HCl
Catalog No.:BCC4362
CAS No.:834-28-6
- PL 017
Catalog No.:BCC5864
CAS No.:83397-56-2
- Prenyl caffeate
Catalog No.:BCC8351
CAS No.:100884-13-7
- D609
Catalog No.:BCC1509
CAS No.:83373-60-8
- 25(R)-Hydroxyprotopanaxadiol
Catalog No.:BCN2493
CAS No.:83349-37-5
- 10-O-Caffeoyl-6-epiferetoside
Catalog No.:BCN4822
CAS No.:83348-22-5
- Dynorphin B
Catalog No.:BCC5987
CAS No.:83335-41-5
- (-)-Lariciresinol
Catalog No.:BCN3418
CAS No.:83327-19-9
- 8(17),13-Labdadien-15,16-olide
Catalog No.:BCN4374
CAS No.:83324-51-0
- Bryostatin 1
Catalog No.:BCC7343
CAS No.:83314-01-6
- 7-Hydroxycoumarin-6-carboxylic acid
Catalog No.:BCN4375
CAS No.:833-52-3
- Ebracteolata cpd B
Catalog No.:BCN3781
CAS No.:83459-37-4
- Ginsenoside Ra1
Catalog No.:BCN8392
CAS No.:83459-41-0
- Mifamurtide
Catalog No.:BCC5241
CAS No.:83461-56-7
- Voglibose
Catalog No.:BCC4750
CAS No.:83480-29-9
- Ginsenoside Rd2
Catalog No.:BCN8279
CAS No.:83480-64-2
- Grantaline
Catalog No.:BCN2083
CAS No.:83482-61-5
- 4-[4-(3-Hydroxyphenyl)-3-(4-methylphenyl)-6-oxo-1,4-dihydropyrrolo[3,4-d]pyrazol-5-yl]benzoic acid
Catalog No.:BCC6341
CAS No.:834903-43-4
- Leucosceptoside A
Catalog No.:BCN7457
CAS No.:83529-62-8
- Anisofolin A
Catalog No.:BCN4377
CAS No.:83529-71-9
- 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane
Catalog No.:BCC8490
CAS No.:83558-87-6
- Regorafenib hydrochloride
Catalog No.:BCC1883
CAS No.:835621-07-3
- Cropodine
Catalog No.:BCN2073
CAS No.:83601-85-8
Synthesis and pharmacological investigation of azaphthalazinone human histamine H(1) receptor antagonists.[Pubmed:22985961]
Bioorg Med Chem. 2012 Oct 15;20(20):6097-108.
5-Aza, 6-aza, 7-aza and 8-aza-phthalazinone, and 5,8-diazaphthalazinone templates were synthesised by stereoselective routes starting from the appropriate pyridine/pyrazine dicarboxylic acids by activation with CDI, reaction with 4-chlorophenyl acetate ester enolate to give a beta-ketoester, which was hydrolysed, and decarboxylated. The resulting ketone was condensed with hydrazine to form the azaphthalazinone core. The azaphthalazinone cores were alkylated with N-Boc-D-Prolinol at N-2 by Mitsunobu reaction, de-protected, and then alkylated at the pyrrolidine nitrogen to provide the target H(1) receptor antagonists. All four mono-azaphthalazinone series had higher affinity (pK(i)) for the human H(1) receptor than azelastine, but were not as potent as the parent non-aza phthalazinone. The 5,8-diazaphthalazinone was equipotent with azelastine. The least potent series were the 7-azaphthalazinones, whereas the 5-azaphthalazinones were the most lipophilic. The more hydrophilic series were the 8-aza series. Replacement of the N-methyl substituent on the pyrrolidine with the n-butyl group caused an increase in potency (pA(2)) and a corresponding increase in lipophilicity. Introduction of a beta-ether oxygen in the n-butyl analogues (2-methoxyethyl group) decreased the H(1) pA(2) slightly, and increased the selectivity against hERG. The duration of action in vitro was longer in the 6-azaphthalazinone series. The more potent and selective 6-azaphthalazinone core was used to append an H(3) receptor antagonist fragment, and to convert the series into the long acting single-ligand, dual H(1) H(3) receptor antagonist 44. The pharmacological profile of 44 was very similar to our intranasal clinical candidate 1.


