CynaropicrinCAS# 35730-78-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35730-78-0 | SDF | Download SDF |
| PubChem ID | 119093 | Appearance | White-beige powder |
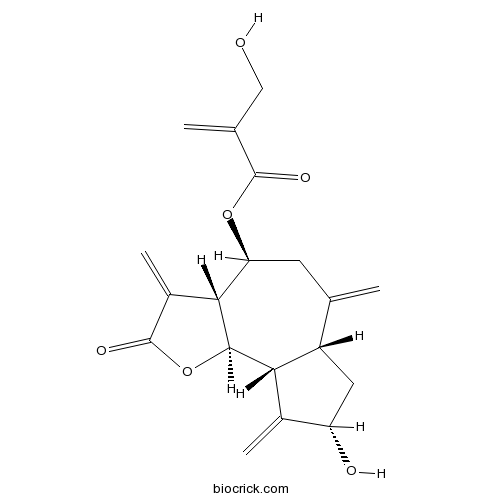
| Formula | C19H22O6 | M.Wt | 346.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (144.35 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(3aR,4S,6aR,8S,9aR,9bR)-8-hydroxy-3,6,9-trimethylidene-2-oxo-3a,4,5,6a,7,8,9a,9b-octahydroazuleno[4,5-b]furan-4-yl] 2-(hydroxymethyl)prop-2-enoate | ||
| SMILES | C=C1CC(C2C(C3C1CC(C3=C)O)OC(=O)C2=C)OC(=O)C(=C)CO | ||
| Standard InChIKey | KHSCYOFDKADJDJ-NQLMQOPMSA-N | ||
| Standard InChI | InChI=1S/C19H22O6/c1-8-5-14(24-18(22)9(2)7-20)16-11(4)19(23)25-17(16)15-10(3)13(21)6-12(8)15/h12-17,20-21H,1-7H2/t12-,13-,14-,15-,16+,17+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cynaropicrin has anti-inflammatory effects, it may participate in the inflammatory response by inhibiting the production of inflammatory mediators and the proliferation of lymphocytes and its inhibitory effect is mediated through conjugation with sulphydryl groups of target protein(s). 2. Cynaropicrin possesses immunomodulatory effects on cytokine release, nitric oxide production and immunosuppressive effects. 3. Cynaropicrin may be a potential anticancer agent against some leukocyte cancer cells such as lymphoma or leukemia, through pro-apoptotic activity. 4. Cynaropicrin shows in vivo activity against Trypanosoma brucei. 5. Cynaropicrin is a potent activator of the AhR-Nrf2-Nqo1 pathway, and could therefore be applied to prevention of UVB-induced photo aging. |
| Targets | TNF-α | NO | IL Receptor | PKC | NADPH-oxidase | Nrf2 |

Cynaropicrin Dilution Calculator

Cynaropicrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8868 mL | 14.4342 mL | 28.8684 mL | 57.7367 mL | 72.1709 mL |
| 5 mM | 0.5774 mL | 2.8868 mL | 5.7737 mL | 11.5473 mL | 14.4342 mL |
| 10 mM | 0.2887 mL | 1.4434 mL | 2.8868 mL | 5.7737 mL | 7.2171 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5774 mL | 1.1547 mL | 1.4434 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2887 mL | 0.5774 mL | 0.7217 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hygrophylline
Catalog No.:BCN2116
CAS No.:3573-82-8
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
- Tropanyl trans-cinnamate
Catalog No.:BCN1931
CAS No.:35721-92-7
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Moslosooflavone
Catalog No.:BCN5301
CAS No.:3570-62-5
- Albaspidin AA
Catalog No.:BCN5300
CAS No.:3570-40-9
- Galantamine
Catalog No.:BCN2868
CAS No.:357-70-0
- Brucine
Catalog No.:BCN2390
CAS No.:357-57-3
- Naloxone HCl
Catalog No.:BCC4612
CAS No.:357-08-4
- Valerenic acid
Catalog No.:BCC7546
CAS No.:3569-10-6
- Questinol
Catalog No.:BCN7443
CAS No.:35688-09-6
- Dihydrobaicalein
Catalog No.:BCN3887
CAS No.:35683-17-1
- Brivaracetam
Catalog No.:BCC5497
CAS No.:357336-20-0
- Fmoc-ß-Ala-OH
Catalog No.:BCC3038
CAS No.:35737-10-1
- Fmoc-Trp-OH
Catalog No.:BCC3556
CAS No.:35737-15-6
- 3beta-Acetoxy-11alpha,12alpha-epoxyoleanan-28,13beta-olide
Catalog No.:BCN6664
CAS No.:35738-25-1
- Oxyimperatorin
Catalog No.:BCN2736
CAS No.:35740-18-2
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
- Cabraleone
Catalog No.:BCN5302
CAS No.:35761-54-7
- N-(3-Hydroxypropyl)-1H-indole-2-carboxamide
Catalog No.:BCC8773
CAS No.:357616-16-1
- Chrysin 7-O-beta-D-glucopyranuronoside
Catalog No.:BCN3249
CAS No.:35775-49-6
- Beta-Hederin
Catalog No.:BCN5381
CAS No.:35790-95-5
- Zapoterin
Catalog No.:BCN5303
CAS No.:35796-71-5
- Sodium Tauroursodeoxycholate (TUDC)
Catalog No.:BCC6516
CAS No.:35807-85-3
Deep Eutectic Solvents as Efficient Media for the Extraction and Recovery of Cynaropicrin from Cynara cardunculus L. Leaves.[Pubmed:29084184]
Int J Mol Sci. 2017 Oct 30;18(11). pii: ijms18112276.
In recent years a high demand for natural ingredients with nutraceutical properties has been witnessed, for which the development of more environmentally-friendly and cost-efficient extraction solvents and methods play a primary role. In this perspective, in this work, the application of deep eutectic solvents (DES), composed of quaternary ammonium salts and organic acids, as alternative solvents for the extraction of Cynaropicrin from Cynara cardunculus L. leaves was studied. After selecting the most promising DES, their aqueous solutions were investigated, allowing to obtain a maximum Cynaropicrin extraction yield of 6.20 wt %, using 70 wt % of water. The sustainability of the extraction process was further optimized by carrying out several extraction cycles, reusing either the biomass or the aqueous solutions of DES. A maximum Cynaropicrin extraction yield of 7.76 wt % by reusing the solvent, and of 8.96 wt % by reusing the biomass, have been obtained. Taking advantage of the Cynaropicrin solubility limit in aqueous solutions, water was added as an anti-solvent, allowing to recover 73.6 wt % of the extracted Cynaropicrin. This work demonstrates the potential of aqueous solutions of DES for the extraction of value-added compounds from biomass and the possible recovery of both the target compounds and solvents.
Haplotype analysis of the germacrene A synthase gene and association with cynaropicrin content and biological activities in Cynara cardunculus.[Pubmed:29143866]
Mol Genet Genomics. 2018 Apr;293(2):417-433.
Cynara cardunculus: L. represents a natural source of terpenic compounds, with the predominant molecule being Cynaropicrin. Cynaropicrin is gaining interest since it has been correlated to anti-hyperlipidaemia, antispasmodic and cytotoxicity activity against leukocyte cancer cells. The objective of this work was to screen a collection of C. cardunculus, from different origins, for new allelic variants in germacrene A synthase (GAS) gene involved in the Cynaropicrin biosynthesis and correlate them with improved Cynaropicrin content and biological activities. Using high-resolution melting, nine haplotypes were identified. The putative impact of the identified allelic variants in GAS protein was evaluated by bioinformatic tools and polymorphisms that putatively lead to protein conformational changes were described. Additionally, Cynaropicrin and main pentacyclic triterpenes contents, and antithrombin, antimicrobial and antiproliferative activities were also determined in C. cardunculus leaf lipophilic-derived extracts. In this work we identified allelic variants with putative impact on GAS protein, which are significantly associated with Cynaropicrin content and antiproliferative activity. The results obtained suggest that the identified polymorphisms should be explored as putative genetic markers correlated with biological properties in Cynara cardunculus.


