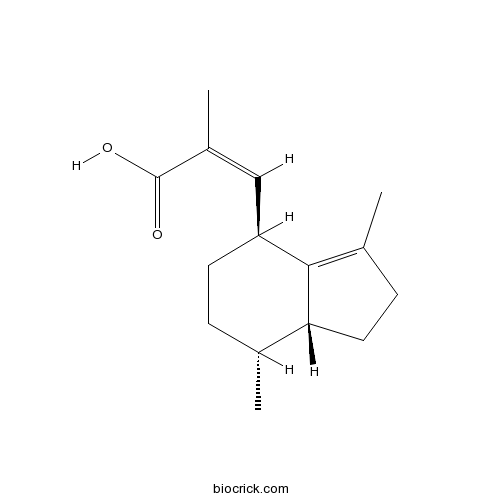
Valerenic acidCAS# 3569-10-6 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3569-10-6 | SDF | Download SDF |
| PubChem ID | 5281538 | Appearance | White powder |
| Formula | C15H22O2 | M.Wt | 234.33 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol | ||
| Chemical Name | (Z)-3-[(4S,7R,7aR)-3,7-dimethyl-2,4,5,6,7,7a-hexahydro-1H-inden-4-yl]-2-methylprop-2-enoic acid | ||
| SMILES | CC1CCC(C2=C(CCC12)C)C=C(C)C(=O)O | ||
| Standard InChIKey | FEBNTWHYQKGEIQ-JWRGBUJQSA-N | ||
| Standard InChI | InChI=1S/C15H22O2/c1-9-4-6-12(8-11(3)15(16)17)14-10(2)5-7-13(9)14/h8-9,12-13H,4-7H2,1-3H3,(H,16,17)/b11-8-/t9-,12+,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator of GABAA receptors that displays preference for receptors containing β2 or β3 subunits. Directly activates the receptor and blocks the channel at high concentrations. Displays sedative, anticonvulsant and anxiolytic activity in vivo. Also acts as a partial agonist of 5-HT5A receptors. |

Valerenic acid Dilution Calculator

Valerenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2675 mL | 21.3374 mL | 42.6749 mL | 85.3497 mL | 106.6872 mL |
| 5 mM | 0.8535 mL | 4.2675 mL | 8.535 mL | 17.0699 mL | 21.3374 mL |
| 10 mM | 0.4267 mL | 2.1337 mL | 4.2675 mL | 8.535 mL | 10.6687 mL |
| 50 mM | 0.0853 mL | 0.4267 mL | 0.8535 mL | 1.707 mL | 2.1337 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4267 mL | 0.8535 mL | 1.0669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Questinol
Catalog No.:BCN7443
CAS No.:35688-09-6
- Dihydrobaicalein
Catalog No.:BCN3887
CAS No.:35683-17-1
- Deoxylapachol
Catalog No.:BCN5299
CAS No.:3568-90-9
- Fmoc-Leu-OH
Catalog No.:BCC3509
CAS No.:35661-60-0
- Fmoc-Phe-OH
Catalog No.:BCC3535
CAS No.:35661-40-6
- Fmoc-Ala-OH
Catalog No.:BCC3034
CAS No.:35661-39-3
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
- Benzbromarone
Catalog No.:BCC4634
CAS No.:3562-84-3
- Mesuaxanthone A
Catalog No.:BCN5298
CAS No.:3561-81-7
- CP 20961
Catalog No.:BCC6063
CAS No.:35607-20-6
- Naloxone HCl
Catalog No.:BCC4612
CAS No.:357-08-4
- Brucine
Catalog No.:BCN2390
CAS No.:357-57-3
- Galantamine
Catalog No.:BCN2868
CAS No.:357-70-0
- Albaspidin AA
Catalog No.:BCN5300
CAS No.:3570-40-9
- Moslosooflavone
Catalog No.:BCN5301
CAS No.:3570-62-5
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Tropanyl trans-cinnamate
Catalog No.:BCN1931
CAS No.:35721-92-7
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
- Hygrophylline
Catalog No.:BCN2116
CAS No.:3573-82-8
- Cynaropicrin
Catalog No.:BCC8161
CAS No.:35730-78-0
- Brivaracetam
Catalog No.:BCC5497
CAS No.:357336-20-0
- Fmoc-ß-Ala-OH
Catalog No.:BCC3038
CAS No.:35737-10-1
Role of 5-HT5A and 5-HT1B/1D receptors in the antinociception produced by ergotamine and valerenic acid in the rat formalin test.[Pubmed:27068146]
Eur J Pharmacol. 2016 Jun 15;781:109-16.
Sumatriptan, dihydroergotamine and methysergide inhibit 1% formalin-induced nociception by activation of peripheral 5-HT1B/1D receptors. This study set out to investigate the pharmacological profile of the antinociception produced by intrathecal and intraplantar administration of ergotamine (a 5-HT1B/1D and 5-HT5A/5B receptor agonist) and Valerenic acid (a partial agonist at 5-HT5A receptors). Intraplantar injection of 1% formalin in the right hind paw resulted in spontaneous flinching behavior of the injected hindpaw of female Wistar rats. Intrathecal ergotamine (15nmol) or Valerenic acid (1 nmol) blocked in a dose dependent manner formalin-induced nociception. The antinociception by intrathecal ergotamine (15nmol) or Valerenic acid (1nmol) was partly or completely blocked by intrathecal administration of the antagonists: (i) methiothepin (non-selective 5-HT5A/5B; 0.01-0.1nmol); (ii) SB-699551 (selective 5-HT5A; up to 10nmol); (iii) anti-5-HT5A antibody; (iv) SB-224289 (selective 5-HT1B; 0.1-1nmol); or (v) BRL-15572 (selective 5-HT1D; 0.1-1nmol). Likewise, antinociception by intraplantar ergotamine (15nmol) and Valerenic acid (10nmol) was: (i) partially blocked by methiothepin (1nmol), SB-699551 (10nmol) or SB-224289 (1nmol); and (ii) abolished by BRL-15572 (1nmol). The above doses of antagonists (which did not affect per se the formalin-induced nociception) were high enough to completely block their respective receptors. Our results suggest that ergotamine and Valerenic acid produce antinociception via 5-HT5A and 5-HT1B/1D receptors located at both spinal and peripheral sites. This provides new evidence for understanding the modulation of nociceptive pathways in inflammatory pain.
Identification of the putative binding pocket of valerenic acid on GABAA receptors using docking studies and site-directed mutagenesis.[Pubmed:26375408]
Br J Pharmacol. 2015 Nov;172(22):5403-13.
BACKGROUND AND PURPOSE: beta2/3-subunit-selective modulation of GABAA receptors by Valerenic acid (VA) is determined by the presence of transmembrane residue beta2/3N265. Currently, it is not known whether beta2/3N265 is part of VA's binding pocket or is involved in the transduction pathway of VA's action. The aim of this study was to clarify the localization of VA's binding pocket on GABAA receptors. EXPERIMENTAL APPROACH: Docking and a structure-based three-dimensional pharmacophore were employed to identify candidate amino acid residues that are likely to interact with VA. Selected amino acid residues were mutated, and VA-induced modulation of the resulting GABAA receptors expressed in Xenopus oocytes was analysed. KEY RESULTS: A binding pocket for VA at the beta(+) /alpha(-) interface encompassing amino acid beta3N265 was predicted. Mutational analysis of suggested amino acid residues revealed a complete loss of VA's activity on beta3M286W channels as well as significantly decreased efficacy and potency of VA on beta3N265S and beta3F289S receptors. In addition, reduced efficacy of VA-induced IGABA enhancement was also observed for alpha1M235W, beta3R269A and beta3M286A constructs. CONCLUSIONS AND IMPLICATIONS: Our data suggest that amino acid residues beta3N265, beta3F289, beta3M286, beta3R269 in the beta3 subunit, at or near the etomidate/propofol binding site(s), form part of a VA binding pocket. The identification of the binding pocket for VA is essential for elucidating its pharmacological effects and might also help to develop new selective GABAA receptor ligands.
Analysis of beta-Subunit-dependent GABAA Receptor Modulation and Behavioral Effects of Valerenic Acid Derivatives.[Pubmed:27190170]
J Pharmacol Exp Ther. 2016 Jun;357(3):580-90.
Valerenic acid (VA)-a beta2/3-selective GABA type A (GABAA) receptor modulator-displays anxiolytic and anticonvulsive effects in mice devoid of sedation, making VA an interesting drug candidate. Here we analyzed beta-subunit-dependent enhancement of GABA-induced chloride currents (IGABA) by a library of VA derivatives and studied their effects on pentylenetetrazole (PTZ)-induced seizure threshold and locomotion. Compound-induced IGABA enhancement was determined in oocytes expressing alpha1beta1gamma2S, alpha1beta2gamma2S, or alpha1beta3gamma2S receptors. Effects on seizure threshold and locomotion were studied using C57BL/6N mice and compared with saline-treated controls. beta2/3-selective VA derivatives such as VA-amide (VA-A) modulating alpha1beta3gamma2S (VA-A: Emax = 972 +/- 69%, n = 6, P < 0.05) and alpha1beta2gamma2S receptors (Emax = 1119 +/- 72%, n = 6, P < 0.05) more efficaciously than VA (alpha1beta3gamma2S: VA: Emax = 632 +/- 88%, n = 9 versus alpha1beta2gamma2S: VA: Emax = 721 +/- 68%, n = 6) displayed significantly more pronounced seizure threshold elevation than VA (saline control: 40.4 +/- 1.4 mg/kg PTZ versus VA 10 mg/kg: 49.0 +/- 1.8 mg/kg PTZ versus VA-A 3 mg/kg: 57.9 +/- 1.9 mg/kg PTZ, P < 0.05). Similarly, VA's methylamide (VA-MA) enhancing IGABA through beta3-containing receptors more efficaciously than VA (Emax = 1043 +/- 57%, P < 0.01, n = 6) displayed stronger anticonvulsive effects. Increased potency of IGABA enhancement and anticonvulsive effects at lower doses compared with VA were observed for VA-tetrazole (alpha1beta3gamma2S: VA-TET: EC50 = 6.0 +/- 1.0 muM, P < 0.05; VA-TET: 0.3 mg/kg: 47.3 +/- 0.5 mg/kg PTZ versus VA: 10 mg/kg: 49.0 +/- 1.8 mg/kg PTZ, P < 0.05). At higher doses (>/=10 mg/kg), VA-A, VA-MA, and VA-TET reduced locomotion. In contrast, unselective VA derivatives induced anticonvulsive effects only at high doses (30 mg/kg) or did not display any behavioral effects. Our data indicate that the beta2/3-selective compounds VA-A, VA-MA, and VA-TET induce anticonvulsive effects at low doses (/=10 mg/kg.
Impact of Altitudes and Habitats on Valerenic Acid, Total Phenolics, Flavonoids, Tannins, and Antioxidant Activity of Valeriana jatamansi.[Pubmed:26971960]
Appl Biochem Biotechnol. 2016 Jul;179(6):911-26.
The changes in total phenolics, flavonoids, tannins, Valerenic acid, and antioxidant activity were assessed in 25 populations of Valeriana jatamansi sampled from 1200 to 2775 m asl and four habitat types of Uttarakhand, West Himalaya. Significant (p < 0.05) variations in total phenolics, flavonoids, Valerenic acid, and antioxidant activity in aerial and root portions and across the populations were observed. Antioxidant activity measured by three in vitro antioxidant assays, i.e., 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic) (ABTS) radical scavenging, 2,2'-diphenyl-1-picryylhydrazyl (DPPH) free radical scavenging, and ferric-reducing antioxidant power (FRAP) assays, showed significant (p < 0.05) differences across the populations. However, no clear pattern was found in phytochemicals across the altitudinal range. Among habitat types, (pine, oak, mixed forest, and grassy land), variation in phytochemical content and antioxidant activity were observed. Equal class ranking, neighbor-joining cluster analysis, and principal component analysis (PCA) identified Talwari, Jaberkhet, Manjkhali, and Khirshu populations as promising sources with higher phytochemicals and antioxidant activity. The results recommended that the identified populations with higher value of phytochemicals and antioxidants can be utilized for mass multiplication and breeding program to meet the domestic as well as commercial demand.


