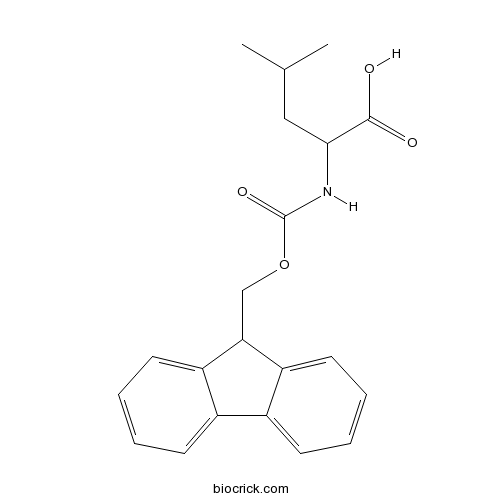
Fmoc-Leu-OHCAS# 35661-60-0 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35661-60-0 | SDF | Download SDF |
| PubChem ID | 4308 | Appearance | Powder |
| Formula | C21H23NO4 | M.Wt | 353.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO with gentle warming | ||
| Chemical Name | 2-(9H-fluoren-9-ylmethoxycarbonylamino)-4-methylpentanoic acid | ||
| SMILES | CC(C)CC(C(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 | ||
| Standard InChIKey | CBPJQFCAFFNICX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H23NO4/c1-13(2)11-19(20(23)24)22-21(25)26-12-18-16-9-5-3-7-14(16)15-8-4-6-10-17(15)18/h3-10,13,18-19H,11-12H2,1-2H3,(H,22,25)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anti-inflammatory agent; increases intracellular Ca2+ levels. |

Fmoc-Leu-OH Dilution Calculator

Fmoc-Leu-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8297 mL | 14.1483 mL | 28.2965 mL | 56.5931 mL | 70.7414 mL |
| 5 mM | 0.5659 mL | 2.8297 mL | 5.6593 mL | 11.3186 mL | 14.1483 mL |
| 10 mM | 0.283 mL | 1.4148 mL | 2.8297 mL | 5.6593 mL | 7.0741 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.5659 mL | 1.1319 mL | 1.4148 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.5659 mL | 0.7074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Leu-OH
- Fmoc-Phe-OH
Catalog No.:BCC3535
CAS No.:35661-40-6
- Fmoc-Ala-OH
Catalog No.:BCC3034
CAS No.:35661-39-3
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
- Benzbromarone
Catalog No.:BCC4634
CAS No.:3562-84-3
- Mesuaxanthone A
Catalog No.:BCN5298
CAS No.:3561-81-7
- CP 20961
Catalog No.:BCC6063
CAS No.:35607-20-6
- N-Desethyl Sunitinib
Catalog No.:BCC1792
CAS No.:356068-97-8
- Toceranib
Catalog No.:BCC2005
CAS No.:356068-94-5
- Darapladib
Catalog No.:BCC1515
CAS No.:356057-34-6
- Fluocinonide
Catalog No.:BCC4953
CAS No.:356-12-7
- Deoxylapachol
Catalog No.:BCN5299
CAS No.:3568-90-9
- Dihydrobaicalein
Catalog No.:BCN3887
CAS No.:35683-17-1
- Questinol
Catalog No.:BCN7443
CAS No.:35688-09-6
- Valerenic acid
Catalog No.:BCC7546
CAS No.:3569-10-6
- Naloxone HCl
Catalog No.:BCC4612
CAS No.:357-08-4
- Brucine
Catalog No.:BCN2390
CAS No.:357-57-3
- Galantamine
Catalog No.:BCN2868
CAS No.:357-70-0
- Albaspidin AA
Catalog No.:BCN5300
CAS No.:3570-40-9
- Moslosooflavone
Catalog No.:BCN5301
CAS No.:3570-62-5
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Tropanyl trans-cinnamate
Catalog No.:BCN1931
CAS No.:35721-92-7
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
NPC-15199, a novel anti-inflammatory agent, mobilizes intracellular Ca2+ in bladder female transitional carcinoma (BFTC) cells.[Pubmed:10857466]
Chin J Physiol. 2000 Mar 31;43(1):29-33.
This report demonstrates that NPC-15199 [(N-(9-fluorenylmethoxycarbonyl)L-leucine)], a novel anti-inflammatory agent, increases intracellular Ca2+ concentration ([Ca2+]i) in human bladder female transitional cancer (BFTC) cells. Using fura-2 as a Ca2+ probe, NPC-15199 (0.1-2 mM) was found to increase [Ca2+]i concentration-dependently. The response saturated at 2-5 mM NPC-15199. The [Ca2+]i increase comprised an initial rise, a slow decay, and a plateau. Ca2+ removal partly inhibited the Ca2+ signals. In Ca2+-free medium, pretreatment with 1 mM NPC-15199 abolished the [Ca2+]i increase induced by 1 microM thapsigargin (an endoplasmic reticulum Ca2+ pump inhibitor); and after pretreatment with thapsigargin, NPC-15199-induced Ca2+ release was dramatically inhibited. This indicates that NPC-15199 released internal Ca2+ mostly from the endoplasmic reticulum. Adding 3 mM Ca2+ increased [Ca2+]i in cells pretreated with 1 mM NPC-15199 in Ca2+-free medium. Together, the findings suggest that in BFTC bladder cancer cells, NPC-15199 induced Ca2+ release from the endoplasmic reticulum and activating Ca2+ entry.
Guinea pig ileitis is attenuated by the leumedin N-(fluorenyl-9- methoxycarbonyl)-leucine (NPC 15199).[Pubmed:8392562]
J Pharmacol Exp Ther. 1993 Jul;266(1):468-72.
Anti-inflammatory properties have been ascribed to a series of N-(fluorenyl-9-methoxycarbonyl) amino acids called leumedins that inhibit the activity of granulocytes and T-lymphocytes. We evaluated one of these leumedins, N-(fluorenyl-9-methoxycarbonyl) leucine (NPC 15199), in a model of ileitis in guinea pigs. Ileitis was induced by intraluminal trinitrobenzenesulfonic acid (TNBS 30 mg/kg in 50% ethanol) in anesthetized guinea pigs. NPC 15199 was administered daily (10 or 100 mg/kg, s.c.). After 7 days, the guinea pigs were anesthetized, and saline was administered intraluminally into an ileal loop created at the site of TNBS administration and was withdrawn after 30 min. The changes in lavage protein, nitrite levels, myeloperoxidase (MPO) activity and mast cell numbers were used as indices of inflammation and injury. NPC 15199 (10 or 100 mg/kg) attenuated or abolished TNBS-induced elevations in lavage protein and nitrite content. Only the high dose of NPC 15199 (100 mg/kg) attenuated ileal MPO activity and mast cell hyperplasia. Histological disturbances induced by TNBS administration included crypt hypertrophy, mucosal and submucosal fibrosis and smooth-muscle hyperplasia. These disturbances were reversed by high-dose NPC 15199 (100 mg/kg) but were minimally affected by low-dose NPC 15199 (10 mg/kg). We conclude that NPC 15199 prevents mucosal injury and dysfunction in this model of intestinal inflammation. Inhibition of granulocyte infiltration does not appear to be essential for the beneficial effects of NPC 15199 and suggests that the alternative actions of NPC 15199 may be pertinent to this model.
N-(fluorenyl-9-methoxycarbonyl) amino acids, a class of antiinflammatory agents with a different mechanism of action.[Pubmed:1824872]
Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):355-9.
Several members of a series of N-(fluorenyl-9-methoxycarbonyl) amino acids were found to possess a broad spectrum of antiinflammatory activity. The compounds were active against oxazolone dermatitis in mice and adjuvant arthritis in rats, models in which activated T lymphocytes are implicated. The compounds also inhibited T-lymphocyte activation in vitro, assessed by using the mixed lymphocyte reaction. The compounds inhibited the reversed passive Arthus reaction in rats and arachidonic acid-induced dermatitis in mice, models in which leukocyte infiltration is responsible for the inflammatory reaction. More complete evaluation was made of one compound, N-(fluorenyl-9-methoxycarbonyl)leucine (NPC 15199). On histologic examination after arachidonic acid administration, NPC 15199 was found to block recruitment of neutrophils into the inflammatory site. The compound was not a general myelotoxin. Prolonged treatment of animals did not alter bone-marrow progenitor number or the numbers of circulating white blood cells. Further, several white cell functions were not inhibited in vitro, including neutrophil respiratory burst and macrophage phagocytosis. NPC 15199 was effective in blocking antigen arthritis in rabbits and was effective in a therapeutic protocol, reversing oxazolone edema. These studies suggest that N-(fluorenyl-9-methoxycarbonyl) amino acids may be valuable therapeutic agents for inflammatory diseases.


