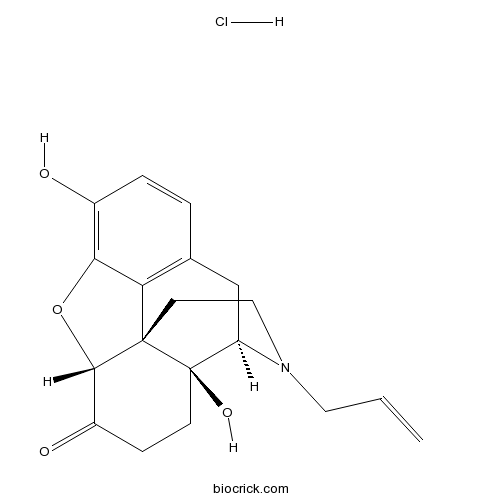
Naloxone HClopioid receptor antagonist, non-selective CAS# 357-08-4 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 357-08-4 | SDF | Download SDF |
| PubChem ID | 5464092 | Appearance | Powder |
| Formula | C19H22ClNO4 | M.Wt | 363.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (5α)-4,5-Epoxy-3,14-dihydro-17-(2-pr | ||
| SMILES | [Cl-].Oc1ccc2C[C@H]3N(CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O)CC=C.[H+] | ||
| Standard InChIKey | RGPDIGOSVORSAK-STHHAXOLSA-N | ||
| Standard InChI | InChI=1S/C19H21NO4.ClH/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11;/h2-4,14,17,21,23H,1,5-10H2;1H/t14-,17+,18+,19-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Opioid antagonist. |

Naloxone HCl Dilution Calculator

Naloxone HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7485 mL | 13.7423 mL | 27.4846 mL | 54.9692 mL | 68.7115 mL |
| 5 mM | 0.5497 mL | 2.7485 mL | 5.4969 mL | 10.9938 mL | 13.7423 mL |
| 10 mM | 0.2748 mL | 1.3742 mL | 2.7485 mL | 5.4969 mL | 6.8712 mL |
| 50 mM | 0.055 mL | 0.2748 mL | 0.5497 mL | 1.0994 mL | 1.3742 mL |
| 100 mM | 0.0275 mL | 0.1374 mL | 0.2748 mL | 0.5497 mL | 0.6871 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KD: 13.1 nM for opioid receptor
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist acting on opioid receptors. Naloxone is commonly used opioid antagonist drug which is a competitive antagonist binding to the opioid receptors with higher affinity than agonists but does not activate the receptors.
In vitro: Naloxone is a non-selective opioid antagonist for the μ-, δ- and κ-opioid receptors. Naloxone competitively inhibits the pharmacologic effects of opioids and, with the classical receptor theory, produces a parallel right shift in the dose-response curves of opioids [1].
In vivo: (or HAD line). Rats of the High Alcohol Drinking (HAD) line were treated with naloxone. Naloxone suppressed water intake when water was presented as the sole source of fluid. In contrast, naloxone produced a dose-dependently decrease in ethanol consumption, without altering water intake. The results suggest activation of the endogenous opioid system may be an important mechanism which serves to maintain continued ethanol drinking [3].
Clinical trial: Naloxone, also known as Narcan among other names, is a medication used to reverse the effects of opioids especially in overdose. The drug was approved for opioid overdose by Food and Drug Administration in 1971.
Reference:
[1] van Dorp E, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf. 2007 Mar;6(2):125-32.
[2] Froehlich JC, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990 Feb;35(2):385-90.
- Valerenic acid
Catalog No.:BCC7546
CAS No.:3569-10-6
- Questinol
Catalog No.:BCN7443
CAS No.:35688-09-6
- Dihydrobaicalein
Catalog No.:BCN3887
CAS No.:35683-17-1
- Deoxylapachol
Catalog No.:BCN5299
CAS No.:3568-90-9
- Fmoc-Leu-OH
Catalog No.:BCC3509
CAS No.:35661-60-0
- Fmoc-Phe-OH
Catalog No.:BCC3535
CAS No.:35661-40-6
- Fmoc-Ala-OH
Catalog No.:BCC3034
CAS No.:35661-39-3
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
- Benzbromarone
Catalog No.:BCC4634
CAS No.:3562-84-3
- Mesuaxanthone A
Catalog No.:BCN5298
CAS No.:3561-81-7
- Brucine
Catalog No.:BCN2390
CAS No.:357-57-3
- Galantamine
Catalog No.:BCN2868
CAS No.:357-70-0
- Albaspidin AA
Catalog No.:BCN5300
CAS No.:3570-40-9
- Moslosooflavone
Catalog No.:BCN5301
CAS No.:3570-62-5
- Pemetrexed disodium hemipenta hydrate
Catalog No.:BCC1844
CAS No.:357166-30-4
- Tropanyl trans-cinnamate
Catalog No.:BCN1931
CAS No.:35721-92-7
- BMS-378806
Catalog No.:BCC4505
CAS No.:357263-13-9
- Hygrophylline
Catalog No.:BCN2116
CAS No.:3573-82-8
- Cynaropicrin
Catalog No.:BCC8161
CAS No.:35730-78-0
- Brivaracetam
Catalog No.:BCC5497
CAS No.:357336-20-0
- Fmoc-ß-Ala-OH
Catalog No.:BCC3038
CAS No.:35737-10-1
- Fmoc-Trp-OH
Catalog No.:BCC3556
CAS No.:35737-15-6
Narcan (naloxone HCl).[Pubmed:2288924]
Gastroenterol Nurs. 1990 Winter;12(3):193-5.
The gastroenterology nurse must be familiar with the use of Narcan. Narcan is frequently administered in the endoscopy suite after the procedure for the reversal of narcotic depression induced by pre-procedure intravenous sedation. Knowledge of Narcan will allow the nurse to safely administer the medication as well as adequately assess the patient's response to it.
Effects of naloxone-HCl on cortisol levels in patients with affective disorder and normal controls.[Pubmed:6943595]
Psychiatry Res. 1981 Jun;4(3):277-83.
Cortisol levels were measured before and after administration of naloxone-HCl in patients with affective disorder (n = 16) and normal control subjects (n = 8). On two consecutive days, 20 mg of naloxone-HCl or placebo was administered i.v. over 15 minutes in a double-blind crossover design. Blood samples were collected at 30, 15, and l minute(s) both before and after infusion. Cortisol rose from a mean baseline level of 14.8 microgram% to a mean peak level of 23.1 microgram% following the naloxone administration. Significant cortisol increases were found in both the 15- and 30-minute samples during the naloxone session. There were no differences between patient and normal subject samples or between diagnostic groups. A subgroup of manic patients who had responded to naloxone with a reduction of their manic behavior also had an attenuated cortisol response to naloxone. This proved to be an artifact secondary to variability in the cortisol response in these patients.
Quantitative evaluation of opioid withdrawal signs in rats repeatedly treated with morphine and injected with naloxone, in the absence or presence of the antiabstinence agent clonidine.[Pubmed:9523765]
J Pharmacol Toxicol Methods. 1997 Nov;38(3):117-31.
An opioid withdrawal syndrome was induced in rats by repeated morphine administration and final naloxone injection. The withdrawal causes alteration of several physiological signs. The aim of the study was to describe a quantitative opioid abstinence syndrome to validate the methodology by utilizing clonidine, a well-known antiwithdrawal agent, and propose the procedure for the screening of antiabstinence drugs. In particular, rats were treated with saline, morphine, naloxone, morphine and naloxone and four doses of clonidine (0, 0.04, 0.1, and 0.25 mg/kg orally). In rats repeatedly exposed to morphine and then injected with naloxone, signs like excretion of feces and urine, salivation, behavioral jumping and wet dog shakes, rectal temperature, and pain threshold have been observed. Consequently, the objective symptoms observed in morphine plus naloxone-treated animals have been taken as markers of opioid withdrawal. These factors have been quantitatively measured and grouped to form a standardized procedure of opioid abstinence syndrome. In addition, it is possible to observe that the antiabstinence drug clonidine exerted effects on modified physiological signs appearing in morphine-dependent rats treated with naloxone, like fecal excretion, levels of rectal temperature, latency times, salivation as well as jumping behavior. The effects exerted by clonidine in this procedure and in other methods are compared and appear to be similar. In addition, comparative observations referring to both the previous methods and the present procedure related to the type of signs studied, the modality of evaluation, and suppressive activity exerted by the antiwithdrawal agent, clonidine, are performed: the greater accuracy of the proposed method becomes apparent. Thus, this experimental model, validated by the antiabstinence agent clonidine, is proposed as a useful screen for drugs affecting opioid withdrawal syndrome.
Naloxone activation of mu-opioid receptors mutated at a histidine residue lining the opioid binding cavity.[Pubmed:9415708]
Mol Pharmacol. 1997 Dec;52(6):983-92.
The mu-opioid receptor is the principal site of action in the brain by which morphine, other opiate drugs of abuse, and endogenous opioid peptides effect analgesia and alter mood. A member of the seven-transmembrane domain (TM) G protein-coupled receptor (GPCR) superfamily, the mu-opioid receptor modulates ion channels and second messenger effectors in an opioid agonist-dependent fashion that is reversible by the classic opiate antagonist naloxone. Mutation of a histidine residue (His297) in TM 6 afforded agonist-like G protein-coupled signal transduction mediated by naloxone and other alkaloid antagonists and enhanced the intrinsic activity of documented alkaloid partial agonists, including buprenorphine. The intrinsic activities of all opioid peptide agonists and antagonists tested were not altered at the His297 mutant receptors. Consistent with a role for the TM 6 histidine in maintaining high affinity binding sites for opioid agonists and antagonists, opioid ligand-dependent protection of this residue from a histidine-specific alkylating agent indicated that the His297 side chain is positioned in or very near the binding cavity. The TM 6 His297 mutants identify a discrete region of the receptor critical for determining whether a specific drug pharmacophore triggers receptor activation. Because many GPCRs possess a similarly positioned TM histidine residue, our findings with the mu-opioid receptor may extend to these receptors and potentially serve as a model for rational design of therapeutic GPCR partial agonists and antagonists.


