Flutax 1Fluorescent taxol derivative CAS# 191930-58-2 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 191930-58-2 | SDF | Download SDF |
| PubChem ID | 16760423 | Appearance | Powder |
| Formula | C71H66N2O21 | M.Wt | 1283.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
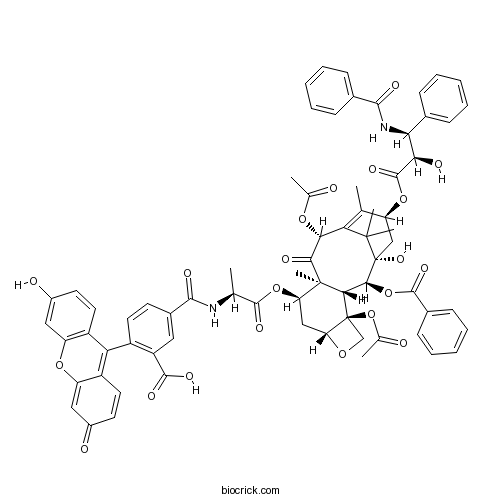
| Chemical Name | 5-[[(2S)-1-[[(1S,2S,3R,4S,7R,9R,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-2-benzoyloxy-1-hydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-9-yl]oxy]-1-oxopropan-2-yl]carbamoyl]-2-(3-hydroxy-6-oxoxanthen-9-yl)benzoic acid | ||
| SMILES | CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C(C(C5=CC=CC=C5)NC(=O)C6=CC=CC=C6)O)O)OC(=O)C7=CC=CC=C7)(CO4)OC(=O)C)OC(=O)C(C)NC(=O)C8=CC(=C(C=C8)C9=C1C=CC(=O)C=C1OC1=C9C=CC(=C1)O)C(=O)O)C)OC(=O)C | ||
| Standard InChIKey | JMWJJMCMRRQCSY-ADONSEPTSA-N | ||
| Standard InChI | InChI=1S/C71H66N2O21/c1-35-51(91-67(86)57(78)56(39-17-11-8-12-18-39)73-62(80)40-19-13-9-14-20-40)33-71(87)61(93-66(85)41-21-15-10-16-22-41)59-69(7,60(79)58(89-37(3)74)55(35)68(71,5)6)52(32-53-70(59,34-88-53)94-38(4)75)92-65(84)36(2)72-63(81)42-23-26-45(48(29-42)64(82)83)54-46-27-24-43(76)30-49(46)90-50-31-44(77)25-28-47(50)54/h8-31,36,51-53,56-59,61,76,78,87H,32-34H2,1-7H3,(H,72,81)(H,73,80)(H,82,83)/t36-,51-,52+,53+,56-,57+,58+,59-,61-,69+,70-,71+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A fluorescent taxol derivative that binds to the taxol microtubule binding site with high affinity (Ka ~ 107M-1). Useful for direct imaging of the microtubule cytoskeleton. Excitation maximum ~ 495 nm; emission maximum ~ 520 nm. |

Flutax 1 Dilution Calculator

Flutax 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.7792 mL | 3.8962 mL | 7.7924 mL | 15.5848 mL | 19.481 mL |
| 5 mM | 0.1558 mL | 0.7792 mL | 1.5585 mL | 3.117 mL | 3.8962 mL |
| 10 mM | 0.0779 mL | 0.3896 mL | 0.7792 mL | 1.5585 mL | 1.9481 mL |
| 50 mM | 0.0156 mL | 0.0779 mL | 0.1558 mL | 0.3117 mL | 0.3896 mL |
| 100 mM | 0.0078 mL | 0.039 mL | 0.0779 mL | 0.1558 mL | 0.1948 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Trimethylgallic acid methyl ester
Catalog No.:BCN3369
CAS No.:1916-07-0
- 2-Deacetoxytaxinine B
Catalog No.:BCN1181
CAS No.:191547-12-3
- Epieriocalyxin A
Catalog No.:BCN1180
CAS No.:191545-24-1
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- MRS 1334
Catalog No.:BCC5753
CAS No.:192053-05-7
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
Comparison of the binding of anti-tubulin antibody and the fluorescent taxol derivative Flutax-1 to the microtubular system of Tetrahymena.[Pubmed:17048696]
Acta Biol Hung. 2006 Sep;57(3):323-9.
Using confocal microscopic analysis, FITC-labelled anti-alpha-tubulin antibody and the fluorescent taxol derivative Flutax-1 in fixed and living Tetrahymena pyriformis GL, longitudinal microtubules, oral and somatic cilia, deep fibers, and contractile vacuole pores were equally labeled. While the antibody stained transversal microtubules, these were not labeled by Flutax-1. At the same time, oral cilia were more intensely stained by Flutax-1, than by the antibody. There were no differences in the staining of fixed preparations and living cells. The observations suggest (i) the difference between the MAPs of longitudinal and transversal microtubules which allow or inhibit the binding of the indicator molecules, and (ii) the different functions of these two types of microtubules.
Fast kinetics of Taxol binding to microtubules. Effects of solution variables and microtubule-associated proteins.[Pubmed:12496245]
J Biol Chem. 2003 Mar 7;278(10):8407-19.
The kinetics of Taxol association to and dissociation from stabilized microtubules has been measured by competition with the reference fluorescent derivative Flutax-1 (Diaz, J. F., Strobe, R., Engelborghs, Y., Souto, A. A., and Andreu, J. M. (2000) J. Biol. Chem. 275, 26265-26276). The association rate constant at 37 degrees C is k(+) = (3.6 +/- 0.1) x 10(6) m(-1) s(-1). The reaction profile is similar to that of the first step of Flutax-1 binding, which probably corresponds to the binding of the Taxol moiety. The rate constant of the initial binding of Flutax-1 is inversely proportional to the viscosity of the solution, which is compatible with a diffusion-controlled reaction. Microtubule-associated proteins bound to the microtubule outer surface slow down the binding of Flutax-1 and Flutax-2 10-fold. The binding site is fully accessible to Flutax-2 in native cytoskeletons of PtK2 cells; the observed kinetic rates of Flutax-2 microtubule staining and de-staining are similar to the reaction rates with microtubule associated proteins-containing microtubules. The kinetic data prove that taxoids bind directly from the bulk solution to an exposed microtubule site. Several hypotheses have been analyzed to potentially reconcile these data with the location of a Taxol-binding site at the model microtubule lumen, including dynamic opening of the microtubule wall and transport from an initial Taxol-binding site at the microtubule pores.
Molecular recognition of taxol by microtubules. Kinetics and thermodynamics of binding of fluorescent taxol derivatives to an exposed site.[Pubmed:10818101]
J Biol Chem. 2000 Aug 25;275(34):26265-76.
We have determined the kinetic scheme and the reaction rates of binding to microtubules of two fluorescent taxoids, 7-O-[N-(4'-fluoresceincarbonyl)-l-alanyl]Taxol (Flutax-1) and 7-O-[N-(2,7-difluoro-4'-fluoresceincarbonyl)-l-alanyl]Taxol (Flutax-2). Flutax-1 and Flutax-2 bind to microtubules with high affinity (K(a) approximately 10(7) m(-1), 37 degrees C). The binding mechanism consists of a fast bimolecular reaction followed by at least two monomolecular rearrangements, which were characterized with stopped-flow techniques. The kinetic constants of the bimolecular reaction were 6.10 +/- 0.22 x 10(5) m(-1) s(-1) and 13.8 +/- 1.8 x 10(5) m(-1) s(-1) at 37 degrees C, respectively. A second slow binding step has been measured employing the change of fluorescence anisotropy of the probe. The reversal of this reaction is the rate-limiting step of dissociation. A third step has been detected using small angle x-ray scattering and involves a 2-nm increase in the diameter of microtubules. It is suggested that the first step entails the binding of the Taxol moiety and the second a relative immobilization of the fluorescent probe. The equilibrium and some kinetic measurements required the use of stabilized cross-linked microtubules, which preserved taxoid binding. The results indicate that the Taxol binding site is directly accessible, in contrast with its location at lumen in the current model of microtubules. An alternative structural model is considered in which the binding site is located between protofilaments, accessible from the microtubule surface.


