HIV-1 Tat Protein PeptideCAS# 191936-91-1 |
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 191936-91-1 | SDF | Download SDF |
| PubChem ID | 25080835 | Appearance | Powder |
| Formula | C64H118N32O14 | M.Wt | 1559.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O Peptide Solubility and Storage Guidelines: 1. Calculate the length of the peptide. 2. Calculate the overall charge of the entire peptide according to the following table: 3. Recommended solution: | ||
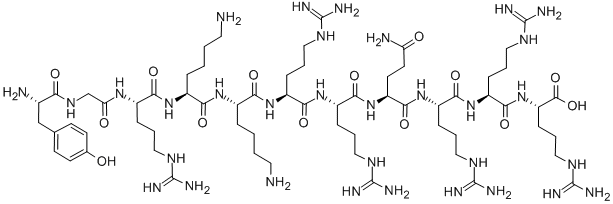
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid | ||
| SMILES | C1=CC(=CC=C1CC(C(=O)NCC(=O)NC(CCCN=C(N)N)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)O)N)O | ||
| Standard InChIKey | RAVVEEJGALCVIN-AGVBWZICSA-N | ||
| Standard InChI | InChI=1S/C64H118N32O14/c65-25-3-1-11-39(89-50(101)38(13-5-27-81-59(69)70)88-48(99)34-87-49(100)37(67)33-35-19-21-36(97)22-20-35)51(102)90-40(12-2-4-26-66)52(103)91-41(14-6-28-82-60(71)72)53(104)92-43(16-8-30-84-62(75)76)55(106)95-45(23-24-47(68)98)57(108)94-42(15-7-29-83-61(73)74)54(105)93-44(17-9-31-85-63(77)78)56(107)96-46(58(109)110)18-10-32-86-64(79)80/h19-22,37-46,97H,1-18,23-34,65-67H2,(H2,68,98)(H,87,100)(H,88,99)(H,89,101)(H,90,102)(H,91,103)(H,92,104)(H,93,105)(H,94,108)(H,95,106)(H,96,107)(H,109,110)(H4,69,70,81)(H4,71,72,82)(H4,73,74,83)(H4,75,76,84)(H4,77,78,85)(H4,79,80,86)/t37-,38-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

HIV-1 Tat Protein Peptide Dilution Calculator

HIV-1 Tat Protein Peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HIV-1 Tat Protein Peptide
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Trimethylgallic acid methyl ester
Catalog No.:BCN3369
CAS No.:1916-07-0
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- MRS 1334
Catalog No.:BCC5753
CAS No.:192053-05-7
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
- Galanganone C
Catalog No.:BCN7486
CAS No.:1922129-46-1
- SIB 1508Y maleate
Catalog No.:BCC7975
CAS No.:192231-16-6
Peptide derived from HIV-1 TAT protein destabilizes a monolayer of endothelial cells in an in vitro model of the blood-brain barrier and allows permeation of high molecular weight proteins.[Pubmed:23150670]
J Biol Chem. 2012 Dec 28;287(53):44676-83.
Most chemotherapeutic agents are blood-brain barrier (BBB) impermeants. HIV-1-derived TAT protein variants contain a transmembrane domain, which may enable them to cross the BBB and reach the brain. Here we synthesized CAYGRKKRRQRRR, a peptide containing a cysteine moiety attached to the N terminus of the transmembrane domain (C-TAT peptide), and studied its effects in an in vitro BBB model, which we found to reflect penetration by a receptor-independent pathway. Incubation of the brain capillary endothelial cell monolayer with 0.3-0.6 mumol/ml of this C-TAT peptide, for a period of 1-2 h, destabilizes brain capillary endothelial cell monolayer and introduces the ability of impermeant therapeutic agents including high molecular weight proteins to penetrate it substantially. The cysteinyl moiety at position 1 of the C-TAT peptide contributes largely to the destabilizing potency and the penetration efficacy of impermeant substances. The destabilizing effect was reversed using heparin. In summary, experimental conditions allowing a significant increase in entry of impermeant low and high molecular weight substances from the luminal (blood) to the abluminal side (brain) were found in an in vitro BBB model reflecting in vivo protein penetrability by a receptor-independent pathway.
Simultaneous recognition of HIV-1 TAR RNA bulge and loop sequences by cyclic peptide mimics of Tat protein.[Pubmed:19584251]
Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):11931-6.
The interaction of the HIV-1 transactivator protein Tat with its transactivation response (TAR) RNA is an essential step in viral replication and therefore an attractive target for developing antivirals with new mechanisms of action. Numerous compounds that bind to the 3-nt bulge responsible for binding Tat have been identified in the past, but none of these molecules had sufficient potency to warrant pharmaceutical development. We have discovered conformationally-constrained cyclic peptide mimetics of Tat that are specific nM inhibitors of the Tat-TAR interaction by using a structure-based approach. The lead peptides are nearly as active as the antiviral drug nevirapine against a variety of clinical isolates in human lymphocytes. The NMR structure of a peptide-RNA complex reveals that these molecules interfere with the recruitment to TAR of both Tat and the essential cellular cofactor transcription elongation factor-b (P-TEFb) by binding simultaneously at the RNA bulge and apical loop, forming an unusually deep pocket. This structure illustrates additional principles in RNA recognition: RNA-binding molecules can achieve specificity by interacting simultaneously with multiple secondary structure elements and by inducing the formation of deep binding pockets in their targets. It also provides insight into the P-TEFb binding site and a rational basis for optimizing the promising antiviral activity observed for these cyclic peptides.
HIV-1 Tat-peptide inhibits protein kinase C and protein kinase A through substrate competition.[Pubmed:20433920]
Eur J Pharm Sci. 2010 Aug 11;40(5):404-11.
HIV-1 Tat-peptide is widely used as a vector for cargo delivery into intact cells. As a cationic, arginine-rich peptide it can readily penetrate the plasma membrane and facilitate the penetration of impermeable bioactive molecules such as proteins, peptides, nucleic acids and drugs. Although at first considered as an inert vector, recent studies have however shown that it might have effects on its own on various cellular processes. In the present study we have investigated the effects of the Tat-peptide(48-60) on two basic serine/threonine kinases, protein kinase C and A, since earlier studies have shown that certain arginine-rich peptides or proteins might have a modulatory effect on their activity. In in vitro studies, Tat-peptide inhibited PKC alpha in a concentration-dependent manner with an IC(50)-value of 22nM and PKA with an IC(50)-value of 1.2 microM. The mode of inhibition was studied in the presence of increasing concentrations of a substrate peptide or ATP. Tat-peptide competed with the kinase substrates, however it did not compete with ATP. In a panel of 70 kinases Tat-peptide showed inhibitory activity at least towards other AGC-family kinases (PKB, SGK1, S6K1, MSK1), CAMK-family kinases (CAMK1 and MELK) and a STE family kinase (MKK1). In HeLa cells Tat-peptide inhibited the phorbol ester-evoked ERK1/2 phosphorylation suggesting that Tat inhibited PKC also in intact cells. In thyroid cells Tat-peptide attenuated sphingosylphosphorylcholine-evoked Ca(2+)-fluxes, which have earlier been shown to be dependent on PKC. Taken together, these results indicate that the Tat-peptide(48-60) is a potent inhibitor which binds to the substrate binding site of the basophilic kinase domain.
Tat peptide-calmodulin binding studies and bioinformatics of HIV-1 protein-calmodulin interactions.[Pubmed:21560167]
Proteins. 2011 Jul;79(7):2233-46.
The human immunodeficiency virus type 1 (HIV-1) genome encodes 18 proteins and 2 peptides. Four of these proteins encode high-affinity calmodulin-binding sites for which direct interactions with calmodulin have already been described. In this study, the HIV-1 proteome is queried using an algorithm that predicts calmodulin-binding sites revealing seven new putative calmodulin-binding sites including residues 34-56 of the transactivator of transcription (Tat). Tat is a 101-residue intrinsically disordered RNA-binding protein that plays a central role in the regulation of HIV-1 replication. Interactions between a Tat peptide (residues 34-56), melittin, a well-characterized calmodulin-binding peptide, and calmodulin were examined by direct binding studies, mass spectrometry, and fluorescence. The Tat peptide binds to both calcium-saturated and apo-calmodulin with a low micromolar affinity. Conformational changes induced in the Tat peptide were determined by circular dichroism, and residues in calmodulin that interact with the peptide were identified by HSQC NMR spectroscopy. Multiple interactions between HIV-1 proteins and calmodulin, a highly promiscuous signal transduction hub protein, may be an important mechanism by which the virus controls cell physiology.


