MRS 1334Potent, highly selective hA3 antagonist CAS# 192053-05-7 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 192053-05-7 | SDF | Download SDF |
| PubChem ID | 4519822 | Appearance | Powder |
| Formula | C31H26N2O6 | M.Wt | 522.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
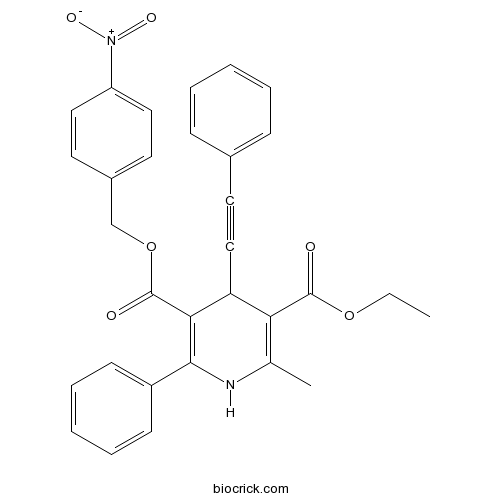
| Chemical Name | 3-O-ethyl 5-O-[(4-nitrophenyl)methyl] 2-methyl-6-phenyl-4-(2-phenylethynyl)-1,4-dihydropyridine-3,5-dicarboxylate | ||
| SMILES | CCOC(=O)C1=C(NC(=C(C1C#CC2=CC=CC=C2)C(=O)OCC3=CC=C(C=C3)[N+](=O)[O-])C4=CC=CC=C4)C | ||
| Standard InChIKey | QFLOJAMZLQXHFS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H26N2O6/c1-3-38-30(34)27-21(2)32-29(24-12-8-5-9-13-24)28(26(27)19-16-22-10-6-4-7-11-22)31(35)39-20-23-14-17-25(18-15-23)33(36)37/h4-15,17-18,26,32H,3,20H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective antagonist for the human adenosine A3 receptor. Ki values are 2.69 nM at hA3, and > 100 μM at rat A1 and rat A2A receptors. |

MRS 1334 Dilution Calculator

MRS 1334 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9137 mL | 9.5683 mL | 19.1366 mL | 38.2731 mL | 47.8414 mL |
| 5 mM | 0.3827 mL | 1.9137 mL | 3.8273 mL | 7.6546 mL | 9.5683 mL |
| 10 mM | 0.1914 mL | 0.9568 mL | 1.9137 mL | 3.8273 mL | 4.7841 mL |
| 50 mM | 0.0383 mL | 0.1914 mL | 0.3827 mL | 0.7655 mL | 0.9568 mL |
| 100 mM | 0.0191 mL | 0.0957 mL | 0.1914 mL | 0.3827 mL | 0.4784 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
- Galanganone C
Catalog No.:BCN7486
CAS No.:1922129-46-1
- SIB 1508Y maleate
Catalog No.:BCC7975
CAS No.:192231-16-6
- CGP 71683 hydrochloride
Catalog No.:BCC7283
CAS No.:192322-50-2
- Prazosin HCl
Catalog No.:BCC2505
CAS No.:19237-84-4
- Neuropeptide AF (human)
Catalog No.:BCC5854
CAS No.:192387-38-5
- Neuropeptide SF (human)
Catalog No.:BCC5829
CAS No.:192387-39-6
Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists.[Pubmed:9703464]
J Med Chem. 1998 Aug 13;41(17):3186-201.
The structure-activity relationships of 6-phenyl-1,4-dihydropyridine derivatives as selective antagonists at human A3 adenosine receptors have been explored (Jiang et al. J. Med. Chem. 1997, 39, 4667-4675). In the present study, related pyridine derivatives have been synthesized and tested for affinity at adenosine receptors in radioligand binding assays. Ki values in the nanomolar range were observed for certain 3,5-diacyl-2,4-dialkyl-6-phenylpyridine derivatives in displacement of [125I]AB-MECA (N6-(4-amino-3-iodobenzyl)-5'-N-methylcarbamoyladenosine) at recombinant human A3 adenosine receptors. Selectivity for A3 adenosine receptors was determined vs radioligand binding at rat brain A1 and A2A receptors. Structure-activity relationships at various positions of the pyridine ring (the 3- and 5-acyl substituents and the 2- and 4-alkyl substituents) were probed. A 4-phenylethynyl group did not enhance A3 selectivity of pyridine derivatives, as it did for the 4-substituted dihydropyridines. At the 2- and 4-positions ethyl was favored over methyl. Also, unlike the dihydropyridines, a thioester group at the 3-position was favored over an ester for affinity at A3 adenosine receptors, and a 5-position benzyl ester decreased affinity. Small cycloalkyl groups at the 6-position of 4-phenylethynyl-1,4-dihydropyridines were favorable for high affinity at human A3 adenosine receptors, while in the pyridine series a 6-cyclopentyl group decreased affinity. 5-Ethyl 2, 4-diethyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate , 38, was highly potent at human A3 receptors, with a Ki value of 20 nM. A 4-propyl derivative, 39b, was selective and highly potent at both human and rat A3 receptors, with Ki values of 18.9 and 113 nM, respectively. A 6-(3-chlorophenyl) derivative, 44, displayed a Ki value of 7.94 nM at human A3 receptors and selectivity of 5200-fold. Molecular modeling, based on the steric and electrostatic alignment (SEAL) method, defined common pharmacophore elements for pyridine and dihydropyridine structures, e.g., the two ester groups and the 6-phenyl group. Moreover, a relationship between affinity and hydrophobicity was found for the pyridines.


