HinokiflavoneCAS# 19202-36-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19202-36-9 | SDF | Download SDF |
| PubChem ID | 5281627 | Appearance | Yellow powder |
| Formula | C30H18O10 | M.Wt | 538.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
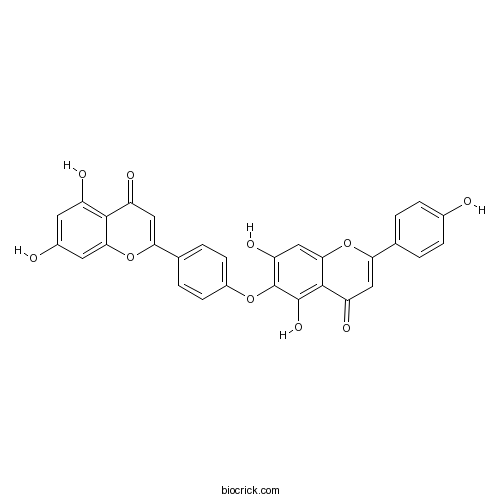
| Chemical Name | 6-[4-(5,7-dihydroxy-4-oxochromen-2-yl)phenoxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(C(=C(C=C3O2)O)OC4=CC=C(C=C4)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O)O | ||
| Standard InChIKey | WTDHMFBJQJSTMH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H18O10/c31-16-5-1-14(2-6-16)24-12-21(35)28-26(40-24)13-22(36)30(29(28)37)38-18-7-3-15(4-8-18)23-11-20(34)27-19(33)9-17(32)10-25(27)39-23/h1-13,31-33,36-37H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Hinokiflavone has significant cytotoxicity, it has inhibition of MMP-9. |
| Targets | MMP(e.g.TIMP) |
| In vitro | Hinokiflavone, a cytotoxic principle from Rhus succedanea and the cytotoxicity of the related biflavonoids.[Pubmed: 2526343]Planta Med. 1989 Apr;55(2):166-8.Hinokiflavone (1) was isolated as the cytotoxic principle from the drupes of Rhus succedanea L.
|
| Kinase Assay | Discovery of potent inhibitor for matrix metalloproteinase-9 by pharmacophore based modeling and dynamics simulation studies.[Pubmed: 24473069]J Mol Graph Model. 2014 Apr;49:25-37.Matrix metalloproteinase-9 (MMP-9) is an attractive target for anticancer therapy.
|

Hinokiflavone Dilution Calculator

Hinokiflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.857 mL | 9.2851 mL | 18.5701 mL | 37.1402 mL | 46.4253 mL |
| 5 mM | 0.3714 mL | 1.857 mL | 3.714 mL | 7.428 mL | 9.2851 mL |
| 10 mM | 0.1857 mL | 0.9285 mL | 1.857 mL | 3.714 mL | 4.6425 mL |
| 50 mM | 0.0371 mL | 0.1857 mL | 0.3714 mL | 0.7428 mL | 0.9285 mL |
| 100 mM | 0.0186 mL | 0.0929 mL | 0.1857 mL | 0.3714 mL | 0.4643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- MRS 1334
Catalog No.:BCC5753
CAS No.:192053-05-7
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
- Galanganone C
Catalog No.:BCN7486
CAS No.:1922129-46-1
- SIB 1508Y maleate
Catalog No.:BCC7975
CAS No.:192231-16-6
- CGP 71683 hydrochloride
Catalog No.:BCC7283
CAS No.:192322-50-2
- Prazosin HCl
Catalog No.:BCC2505
CAS No.:19237-84-4
Hinokiflavone, a cytotoxic principle from Rhus succedanea and the cytotoxicity of the related biflavonoids.[Pubmed:2526343]
Planta Med. 1989 Apr;55(2):166-8.
Hinokiflavone (1) was isolated as the cytotoxic principle from the drupes of Rhus succedanea L. A comparison of the cytotoxicity of 1 and other related biflavonoids, including amentoflavone (2), robustaflavone (3), agathisflavone (4), rhusflavone (5), rhusflavanone (6) and its hexaacetate (7), succedaneaflavanone (8) and its hexaacetate (9), cupressuflavone (10), neorhusflavanone (11), volkensiflavone (12) and its hexamethyl ether (13), spicataside (14) and its nonaacetate (15), morelloflavone (16) and its heptaacetate (17) and heptamethyl ether (18), GB-1a (19) and its hexamethyl ether (20) and 7"-O-beta-glucoside (21), and GB-2a (22), indicates that an ether linkage between two units of apigenin as seen in 1 is structurally required for significant cytotoxicity. Compounds 13 and 20 also demonstrated significant cytotoxicity.
Discovery of potent inhibitor for matrix metalloproteinase-9 by pharmacophore based modeling and dynamics simulation studies.[Pubmed:24473069]
J Mol Graph Model. 2014 Apr;49:25-37.
Matrix metalloproteinase-9 (MMP-9) is an attractive target for anticancer therapy. In the present study ligand based pharmacophore modeling was performed to elucidate the structural elements for a diverse class of MMP-9 inhibitors. The pharmacophore model was validated through Guner-Henry (GH) scoring method. The final pharmacophore model consisted of three hydrogen bond acceptors (HBA), and two ring aromatic regions (RA). This model was utilized to screen the natural compound database to seek novel compounds as MMP-9 inhibitors. The identified hits were validated using molecular docking and molecular dynamics simulation studies. Finally, one compound named Hinokiflavone from Juniperus communis had high binding free energy of -26.54kJ/mol compared with the known inhibitors of MMP-9. Cytotoxicity for Hinokiflavone was evaluated by MTT assay. Inhibition of MMP-9 in the presence of Hinokiflavone was detected by gelatin zymography and gelatinolytic inhibition assay. Results revealed that the natural compounds derived based on the developed pharmacophore model would be useful for further design and development of MMP-9 inhibitors.


