Cyclo(Pro-Gly)CAS# 19179-12-5 |
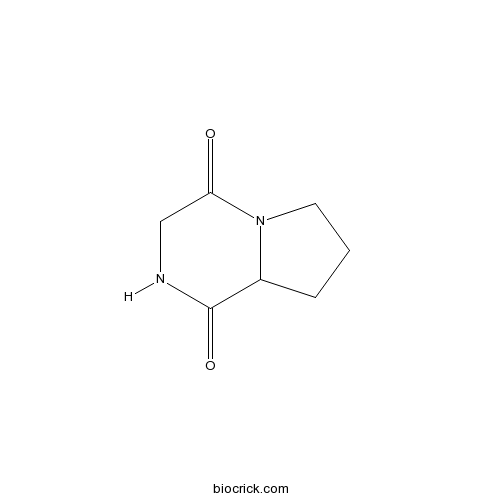
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19179-12-5 | SDF | Download SDF |
| PubChem ID | 193540 | Appearance | Powder |
| Formula | C7H10N2O2 | M.Wt | 154.17 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | C1CC2C(=O)NCC(=O)N2C1 | ||
| Standard InChIKey | OWOHLURDBZHNGG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H10N2O2/c10-6-4-8-7(11)5-2-1-3-9(5)6/h5H,1-4H2,(H,8,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(Pro-Gly) is an active metabolite of piracetam-N-phenylacetyl-L-prolylglycine (GWS-111), it shows a greater resistance to an enzymatic effect than natural neuropeptides. Cyclo-(Gly-Pro) shows cytotoxicity at the concentration of 10 umol/L, it inhibits the growth of Bacillus subtilis with the minimal inhibitory concentration (MIC) value of 0.8, 0.8 g/L. |
| Targets | Antifection |
| In vitro | The Endophytic Fungus Strain FTJZZJ09 Isolated from the Bulbs of Fritillaria thunbergii and Its Antibacterial Metabolites[Reference: WebLink]Microbiology China,2010, 37(10):1475-80.
The antitumor active component from marine derived actinomycete S1001[Reference: WebLink]Chinese Journal of Antibiotics, 2006, 31(1):36-38,62.
|
| Structure Identification | Journal of Mass Spectrometry Volume 33, Issue 1, pages 25–34, January 1998Energetics of the cyclo(Pro–Gly) cation fragmentation: a mass spectrometric study and theoretical calculations[Reference: WebLink]
|

Cyclo(Pro-Gly) Dilution Calculator

Cyclo(Pro-Gly) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4863 mL | 32.4317 mL | 64.8635 mL | 129.7269 mL | 162.1587 mL |
| 5 mM | 1.2973 mL | 6.4863 mL | 12.9727 mL | 25.9454 mL | 32.4317 mL |
| 10 mM | 0.6486 mL | 3.2432 mL | 6.4863 mL | 12.9727 mL | 16.2159 mL |
| 50 mM | 0.1297 mL | 0.6486 mL | 1.2973 mL | 2.5945 mL | 3.2432 mL |
| 100 mM | 0.0649 mL | 0.3243 mL | 0.6486 mL | 1.2973 mL | 1.6216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Trimethylgallic acid methyl ester
Catalog No.:BCN3369
CAS No.:1916-07-0
- 2-Deacetoxytaxinine B
Catalog No.:BCN1181
CAS No.:191547-12-3
- Epieriocalyxin A
Catalog No.:BCN1180
CAS No.:191545-24-1
- 2-Hydroxyxanthone
Catalog No.:BCN7545
CAS No.:1915-98-6
- Isoficusin A
Catalog No.:BCN6865
CAS No.:1914963-20-4
- LY 379268
Catalog No.:BCC7368
CAS No.:191471-52-0
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- 12-Hydroxymyricanone
Catalog No.:BCN8046
CAS No.:191999-68-5
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- MRS 1334
Catalog No.:BCC5753
CAS No.:192053-05-7
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
Streptomyces nigra sp. nov. Is a Novel Actinobacterium Isolated From Mangrove Soil and Exerts a Potent Antitumor Activity in Vitro.[Pubmed:30072967]
Front Microbiol. 2018 Jul 18;9:1587.
A new bacterial strain, designated 452(T), was isolated from the rhizosphere soil of the mangrove Avicennia marina in China. As determined, its cell wall peptidoglycan contained LL-diaminopimelic acid; MK-9(H8) and MK-9(H6) were the major isoprenoid quinones; and iso-C16:0 (31.3%), anteiso-C15:0 (16.9%), and iso-C15:0 (12.5%) were the major cellular fatty acids (>10.0%). Phylogenetic analysis based on the 16S rRNA gene sequence revealed that strain 452(T) formed a distinct lineage in the clade of the genus Streptomyces, and was closely related to S. coerulescens DSM 40146(T) (99.6% sequence identity), S. bellus DSM 40185(T) (99.5%), and S. coeruleorubidus DSM 41172(T) (99.3%). The DNA-DNA relatedness between strain 452(T) and these type strains ranged between 29.3 and 42.3%. Based on the phenotypic, chemotaxonomic, and phylogenetic features, the strain 452(T) is considered to represent a novel species of the genus Streptomyces, for which the name Streptomyces nigra sp. nov. is proposed. The type strain is 452(T) (=KCTC 39960(T) = MCCC 1K03346(T)). Further, strain 452(T) extracts exhibited a pronounced antitumor activity against human cancer cell lines A549, HCT-116, and HepG2, but not against normal human colon cells CCD-18Co. Active substances in the fermentation broth of strain 452(T) were isolated by bioassay-guided analysis, and then purified using a macroporous resin, silica gel, sephadex LX-20 column, and semi-preparative high-performance liquid chromatography (HPLC). Eight proline-containing diketopiperazines, namely, cyclo(Pro-Ala), Cyclo(Pro-Gly), cyclo(Pro-Phe), cyclo(Pro-Met), cyclo(Pro-Val), cyclo(Pro-Leu), cyclo(Pro-Tyr), and cyclo(L-Leu-trans-4-hydroxy-L-Pro), were identified by electrospray ionization mass spectrometry (MS) and nuclear magnetic resonance (NMR). The compounds displayed different levels of cytotoxicity. The highest cytotoxicity was exhibited by cyclo(Pro-Ala) and cyclo(Pro-Met) against A549 cells, and cyclo(Phe-Pro) and cyclo(Pro-Ala) against HCT-116 cells, with average IC50 values equal to 18.5, 27.3, 32.3, and 47.6 mug/mL, respectively. The diversity of diketopiperazines and other chemicals produced by 452(T) was further investigated using gas chromatography (GC)-MS and liquid chromatography (LC)-MS. The analysis revealed 16 types of metabolites with antitumor activity and 16 other types of diketopiperazines. Hence, extracts of the newly identified strain may be used a starting material for the development of antitumor agents.
Chiral Recognition of Diketopiperazine Cyclo(Pro-Gly) and Propranolol Using (-)-Epigallocatechin-3-O-gallate.[Pubmed:26833443]
Chem Pharm Bull (Tokyo). 2016;64(2):142-9.
In the (1)H-NMR spectrum of a solution containing an equimolecular amount of cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) and (-)-epigallocatechin-3-O-gallate (EGCg) in a D2O, a difference in the chemical shift of (1)H-NMR signal for H7alpha, H7beta,8alpha of the Pro residue was observed. Judging from the crystal structures of the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), the difference in the chemical shift resulted mainly from a magnetic anisotropic shielding effect by the ring current from the B ring of EGCg. Therefore, it was considered that chirality of Cyclo(Pro-Gly) was recognized by EGCg in the D2O solution. Furthermore, in the (1)H-NMR spectrum of a solution containing an equimolecular amount of racemic propranolol ((R)- and (S)-propranolols) and EGCg in D2O, the (1)H-NMR signal for H2 of the naphthalene group was observed as two doublets, suggesting that the racemic propranolol formed diastereomers of complexes with EGCg; as a result, chirality of propranolol was recognized by EGCg in the D2O solution.


