MethacrylamideCAS# 79-39-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79-39-0 | SDF | Download SDF |
| PubChem ID | 6595 | Appearance | Powder |
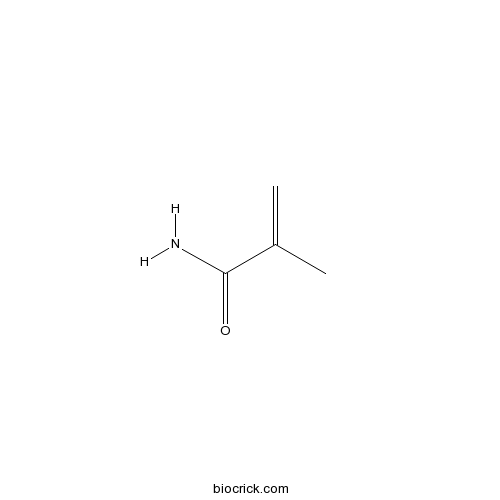
| Formula | C4H7NO | M.Wt | 85.10 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-methylprop-2-enamide | ||
| SMILES | CC(=C)C(=O)N | ||
| Standard InChIKey | FQPSGWSUVKBHSU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Reactive comonomer for acrylic resins. |

Methacrylamide Dilution Calculator

Methacrylamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 11.7509 mL | 58.7544 mL | 117.5088 mL | 235.0176 mL | 293.772 mL |
| 5 mM | 2.3502 mL | 11.7509 mL | 23.5018 mL | 47.0035 mL | 58.7544 mL |
| 10 mM | 1.1751 mL | 5.8754 mL | 11.7509 mL | 23.5018 mL | 29.3772 mL |
| 50 mM | 0.235 mL | 1.1751 mL | 2.3502 mL | 4.7004 mL | 5.8754 mL |
| 100 mM | 0.1175 mL | 0.5875 mL | 1.1751 mL | 2.3502 mL | 2.9377 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Guan-fu base G
Catalog No.:BCN8493
CAS No.:78969-72-9
- DL-AP7
Catalog No.:BCC6551
CAS No.:78966-69-5
- L-AP6
Catalog No.:BCC6612
CAS No.:78944-89-5
- Chicanine
Catalog No.:BCN7818
CAS No.:78919-28-5
- Iloprost
Catalog No.:BCC7247
CAS No.:78919-13-8
- Deacetylnimbinene
Catalog No.:BCN4578
CAS No.:912545-53-0
- Orobanone
Catalog No.:BCN3562
CAS No.:78916-35-5
- 6-Thio-dG
Catalog No.:BCC6507
CAS No.:789-61-7
- 1-chloro-6-(5-ethynylthiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1351
CAS No.:78876-53-6
- 1-chloro-6-(5-(prop-1-ynyl)thiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1352
CAS No.:78876-52-5
- Demethylasterriquinone B1
Catalog No.:BCC7189
CAS No.:78860-34-1
- Garcinol
Catalog No.:BCC5623
CAS No.:78824-30-3
- Oxytetracycline (Terramycin)
Catalog No.:BCC4819
CAS No.:79-57-2
- Lanosterol
Catalog No.:BCN3332
CAS No.:79-63-0
- Retinyl (Vitamin A) Palmitate
Catalog No.:BCC4749
CAS No.:79-81-2
- Camphene
Catalog No.:BCC9217
CAS No.:79-92-5
- 4'-O-Methylnyasol
Catalog No.:BCN7564
CAS No.:79004-25-4
- Eurycomalin A
Catalog No.:BCN3654
CAS No.:790234-20-7
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
- SNAP
Catalog No.:BCC6712
CAS No.:79032-48-7
- β-Estradiol - d3
Catalog No.:BCC5365
CAS No.:79037-37-9
- L-AP5
Catalog No.:BCC6554
CAS No.:79055-67-7
- D-AP5
Catalog No.:BCC6553
CAS No.:79055-68-8
- Boc-Alaninol
Catalog No.:BCC2730
CAS No.:79069-13-9
Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan beads.[Pubmed:29746971]
Int J Biol Macromol. 2018 Sep;116:255-263.
In this study, the Cu(II) and Cd(II) ions removal behavior of crosslinked chitosan beads grafted poly(Methacrylamide) (abbreviated as crosslinked chitosan-g-PMAm) from single metal ion solutions was investigated. The modified chitosan beads presented a remarkable improvement in acid resistance. The batch experiments demonstrated that pH of solution played a significant role in adsorption. It was found that the adsorption of Cu(II) and Cd(II) were optimum at pH4 and pH5, respectively. The maximum adsorption capacities for Cu(II) and Cd(II) based on Langmuir equation were 140.9mgg(-1) and 178.6mgg(-1), respectively. Pseudo-second order gave a better fit for adsorption data with respect to linearity coefficients than pseudo-first order suggesting that chemisorption or electron transfer is the dominant mechanism of the metal ions onto crosslinked chitosan-g-PMAm. In addition, X-ray photoelectron spectroscopy (XPS) investigations revealed that adsorption of both metal ions took place on the surfaces of crosslinked chitosan-g-PMAm by chelation through CNH2, CO and CO groups. Overall, the modified chitosan has proved a promising adsorbent for removal of metal ions.
Influence of dynamic flow conditions on adsorbed plasma protein corona and surface-induced thrombus generation on antifouling brushes.[Pubmed:29549767]
Biomaterials. 2018 Jun;166:79-95.
The information regarding the nature of protein corona (and its changes) and cell binding on biomaterial surface under dynamic conditions is critical to dissect the mechanism of surface-induced thrombosis. In this manuscript, we investigated the nature of protein corona and blood cell binding in heparinized recalcified human plasma, platelet rich plasma and whole blood on three highly hydrophilic antifouling polymer brushes, (poly(N, N-dimethylacrylamide) (PDMA), poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and poly[N-(2-hydroxypropyl) Methacrylamide] (PHPMA) using an in vitro blood loop model at comparable arterial and venous flow, and static conditions. A fluid dynamics model was used initially to better understand the resulting flow patterns in a vertical channel containing the substrates to arrive at the placement of the substrates within the blood loop. The protein binding on the brush modified substrates was determined using ellipsometry, fluorescence microscopy and the nature of the protein corona was investigated using mass spectrometry based proteomics. The flow elevated fouling on brush coated surface from blood. The extent of plasma protein adsorption and platelet adhesion onto PDMA brush was lower than other surfaces in both static and flow conditions. The profiles of adsorbed protein corona showed strong dependence on the test conditions (static vs. flow), and the chemistry of the polymer brushes. Specially, the PDMA brush under flow conditions was more enriched with coagulation proteins, complement proteins, vitronectin and fibronectin but was less enriched with serum albumin. Apolipoprotein B-100 and complement proteins were the most abundant proteins seen on PMPC and PHPMA surfaces under both flow and static conditions, respectively. Unlike PDMA brush, the flow conditions did not affect the composition of protein corona on PMPC and PHPMA brushes. The nature of the protein corona formed in flow conditions influenced the platelet and red blood cell binding. The dependence of shear stress on platelet adhesion from platelet rich plasma and whole blood highlights the contribution of red blood cells in enhancing platelet adhesion on the surface under high shear condition.
Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer-Doxorubicin Drug Conjugates with pH-Triggered Drug Release.[Pubmed:29543465]
Mol Pharm. 2018 Sep 4;15(9):3654-3663.
Herein, the biodegradable micelle-forming amphiphilic N-(2-hydroxypropyl) Methacrylamide (HPMA)-based polymer conjugates with the anticancer drug doxorubicin (Dox) designed for enhanced tumor accumulation were investigated, and the influence of their stability in the bloodstream on biodistribution, namely, tumor uptake, and in vivo antitumor efficacy were evaluated in detail. Dox was attached to the polymer carrier by a hydrazone bond enabling pH-controlled drug release. While the polymer-drug conjugates were stable in a buffer at pH 7.4 (mimicking bloodstream environment), Dox was released in a buffer under mild acidic conditions modeling the tumor microenvironment or cells. The amphiphilic polymer carriers differed in the structure of hydrophobic cholesterol derivative moieties bound to the HPMA copolymers via a hydrolyzable hydrazone bond, exhibiting different rates of micellar structure disintegration at various pH values. Considerable dependence of the studied polymer-drug conjugate biodistribution on the stability of the micellar structure was observed in neutral, bloodstream-mimicking, environment, showing that a faster rate of the micelle disintegration in pH 7.4 increased the conjugate blood clearance, decreased tumor accumulation, and significantly reduced the tumor:blood and tumor:muscle ratios. Similarly, the final therapeutic outcome was strongly affected by the stability of the micellar structure because the most stable micellar conjugate showed an almost similar therapeutic outcome as the water-soluble, nondegradable, high-molecular-weight starlike HPMA copolymer-Dox conjugate, which was highly efficient in the treatment of solid tumors in mice. Based on the results, we conclude that the bloodstream stability of micellar polymer-anticancer drug conjugates, in addition to their low side toxicity, is a crucial parameter for their efficient solid tumor accumulation and high in vivo antitumor activity.
Tumor Specific and Renal Excretable Star-like Triblock Polymer-Doxorubicin Conjugates for Safe and Efficient Anticancer Therapy.[Pubmed:29742345]
Biomacromolecules. 2018 Jul 9;19(7):2849-2862.
Efficient tumor accumulation and body clearance are two paralleled requirements for ideal nanomedicines. However, it is hard for both to be met simultaneously. The inefficient clearance often restrains the application of drug delivery systems (DDSs), especially for high-dosage administration. In this study, the star-like and block structures are combined to enhance the tumor specific targeting of the parent structures and obtain additional renal excretion property. The influences of polymer architectures and chemical compositions on the physicochemical and biological properties, particularly the simultaneous achievement of tumor accumulation and renal clearance, have been investigated. Among the tested conjugates, an eight-arm triblock star polymer based on poly(ethylene glycol) (PEG) and poly( N-(2-hydroxyl) Methacrylamide) (PHPMA) is found to simultaneously fulfill the requirements of superior tumor accumulation and efficient renal clearance due to the appropriate micelle size and reversible aggregation process. On the basis of this conjugate, 60 mg/kg of Dox equivalent (much higher than the maximum tolerated dose (MTD) of Dox) can be administered to efficiently suppress tumor growth without causing any obvious toxicity. This work provides a new approach to design polymer-drug conjugates for tumor specific application, which can simultaneously address the efficacy and safety concerns.
Enzyme/pH-sensitive polyHPMA-DOX conjugate as a biocompatible and efficient anticancer agent.[Pubmed:29564431]
Biomater Sci. 2018 May 1;6(5):1177-1188.
In this study, to enhance the therapeutic function and reduce the side-effects of doxorubicin (DOX), a biodegradable N-(2-hydroxypropyl) Methacrylamide (HPMA) polymer-DOX conjugate has been prepared through reversible addition fragmentation chain transfer (RAFT) polymerization and conjugation chemistry, and the anticancer agent DOX was covalently linked to the polymeric vehicle through a pH-responsive hydrazone bond. The cellular mechanisms of the conjugate were explored, and the therapeutic indexes were studied as well. The high molecular weight (MW) polymeric conjugate (94 kDa) was degraded into products with low MW (45 kDa) in the presence of lysosomal cathepsin B and also showed pH-responsive drug release behavior. In vitro cellular mechanism studies revealed that the polymeric conjugate was uptaken by the 4T1 cells, leading to cell apoptosis and cytotoxicity to cancer cells, while the polymeric conjugate demonstrated excellent in vivo biosafety even at a high dose. Compared to free DOX, the conjugate has a much longer half-life in pharmacokinetics and accumulates in tumors with a much higher amount. The conjugate therefore has a much greater in vivo anticancer efficacy against 4T1 xenograft tumors and shows subtle side-effects, which were confirmed via tumor size and weight, immunohistochemistry and histological studies. Overall, this polymeric conjugate may be used as an enzyme/pH-sensitive anticancer agent.
Synergistic effect of kappa-carrageenan and gelatin blends towards adipose tissue engineering.[Pubmed:29580385]
Carbohydr Polym. 2018 Jun 1;189:1-9.
The current paper focuses on the functionalization of kappa-carrageenan and gelatin as extracellular matrix polysaccharide and protein mimic respectively to produce hydrogel films for adipose tissue engineering. More specifically, kappa-carrageenan as well as gelatin have been functionalized with methacrylate and Methacrylamide moieties respectively to enable subsequent UV-induced crosslinking in the presence of a photo-initiator. The gel fraction, the mass swelling ratio and the mechanical properties of both the one-component hydrogels and the protein/polysaccharide blends have been evaluated. The mechanical and swelling properties of the blends could be tuned by varying the hydrogel composition as well as the crosslinking method applied. The in vitro biocompatibility assays indicated a significantly higher cell viability of adipose tissue-derived mesenchymal stem cells seeded onto the blends as compared to the one-component hydrogels. The results show that the blends of gelatin and kappa-carrageenan clearly outperform the one-component hydrogels in terms of adipose tissue engineering potential.
Interaction of spin-labeled HPMA-based nanoparticles with human blood plasma proteins - the introduction of protein-corona-free polymer nanomedicine.[Pubmed:29560983]
Nanoscale. 2018 Mar 29;10(13):6194-6204.
In this paper, we revised the current understanding of the protein corona that is created on the surface of nanoparticles in blood plasma after an intravenous injection. We have focused on nanoparticles that have a proven therapeutic outcome. These nanoparticles are based on two types of biocompatible amphiphilic copolymers based on N-(2-hydroxypropyl)Methacrylamide (HPMA): a block copolymer, poly(epsilon-caprolactone) (PCL)-b-poly(HPMA), and a statistical HPMA copolymer bearing cholesterol moieties, which have been tested both in vitro and in vivo. We studied the interaction of nanoparticles with blood plasma and selected blood plasma proteins by electron paramagnetic resonance (EPR), isothermal titration calorimetry, dynamic light scattering, and cryo-transmission electron microscopy. The copolymers were labeled with TEMPO radicals at the end of hydrophobic PCL or along the hydrophilic HPMA chains to monitor changes in polymer chain dynamics caused by protein adsorption. By EPR and other methods, we were able to probe specific interactions between nanoparticles and blood proteins, specifically low- and high-density lipoproteins, immunoglobulin G, human serum albumin (HSA), and human plasma. It was found that individual proteins and plasma have very low binding affinity to nanoparticles. We observed no hard corona around HPMA-based nanoparticles; with the exception of HSA the proteins showed no detectable binding to the nanoparticles. Our study confirms that a classical "hard corona-soft corona" paradigm is not valid for all types of nanoparticles and each system has a unique protein corona that is determined by the nature of the NP material.
Histidine-rich glycoprotein-induced vascular normalization improves EPR-mediated drug targeting to and into tumors.[Pubmed:29730154]
J Control Release. 2018 Jul 28;282:25-34.
Tumors are characterized by leaky blood vessels, and by an abnormal and heterogeneous vascular network. These pathophysiological characteristics contribute to the enhanced permeability and retention (EPR) effect, which is one of the key rationales for developing tumor-targeted drug delivery systems. Vessel abnormality and heterogeneity, however, which typically result from excessive pro-angiogenic signaling, can also hinder efficient drug delivery to and into tumors. Using histidine-rich glycoprotein (HRG) knockout and wild type mice, and HRG-overexpressing and normal t241 fibrosarcoma cells, we evaluated the effect of genetically induced and macrophage-mediated vascular normalization on the tumor accumulation and penetration of 10-20nm-sized polymeric drug carriers based on poly(N-(2-hydroxypropyl)Methacrylamide). Multimodal and multiscale optical imaging was employed to show that normalizing the tumor vasculature improves the accumulation of fluorophore-labeled polymers in tumors, and promotes their penetration out of tumor blood vessels deep into the interstitium.


