DL-AP7CAS# 78966-69-5 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78966-69-5 | SDF | Download SDF |
| PubChem ID | 1617429 | Appearance | Powder |
| Formula | C7H16NO5P | M.Wt | 225.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
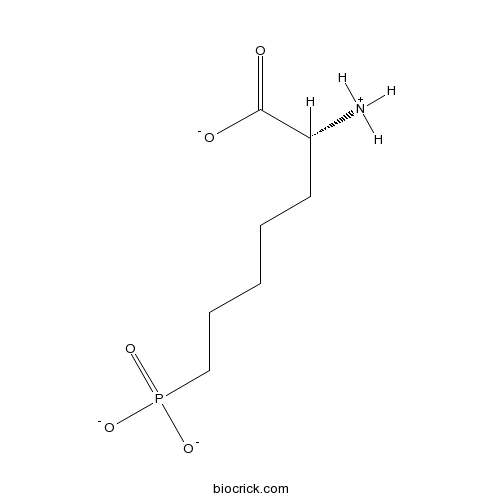
| Chemical Name | (2R)-2-azaniumyl-7-phosphonatoheptanoate | ||
| SMILES | C(CCC(C(=O)[O-])[NH3+])CCP(=O)([O-])[O-] | ||
| Standard InChIKey | MYDMWESTDPJANS-ZCFIWIBFSA-L | ||
| Standard InChI | InChI=1S/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13)/p-2/t6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | First generation phosphono NMDA antagonist. Anticonvulsant. |

DL-AP7 Dilution Calculator

DL-AP7 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4409 mL | 22.2045 mL | 44.4089 mL | 88.8178 mL | 111.0223 mL |
| 5 mM | 0.8882 mL | 4.4409 mL | 8.8818 mL | 17.7636 mL | 22.2045 mL |
| 10 mM | 0.4441 mL | 2.2204 mL | 4.4409 mL | 8.8818 mL | 11.1022 mL |
| 50 mM | 0.0888 mL | 0.4441 mL | 0.8882 mL | 1.7764 mL | 2.2204 mL |
| 100 mM | 0.0444 mL | 0.222 mL | 0.4441 mL | 0.8882 mL | 1.1102 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-AP6
Catalog No.:BCC6612
CAS No.:78944-89-5
- Chicanine
Catalog No.:BCN7818
CAS No.:78919-28-5
- Iloprost
Catalog No.:BCC7247
CAS No.:78919-13-8
- Deacetylnimbinene
Catalog No.:BCN4578
CAS No.:912545-53-0
- Orobanone
Catalog No.:BCN3562
CAS No.:78916-35-5
- 6-Thio-dG
Catalog No.:BCC6507
CAS No.:789-61-7
- 1-chloro-6-(5-ethynylthiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1351
CAS No.:78876-53-6
- 1-chloro-6-(5-(prop-1-ynyl)thiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1352
CAS No.:78876-52-5
- Demethylasterriquinone B1
Catalog No.:BCC7189
CAS No.:78860-34-1
- Garcinol
Catalog No.:BCC5623
CAS No.:78824-30-3
- Epibrassinolide
Catalog No.:BCC5479
CAS No.:78821-43-9
- 4-Benzyloxycarbonyl-2-piperazinone
Catalog No.:BCC8699
CAS No.:78818-15-2
- Guan-fu base G
Catalog No.:BCN8493
CAS No.:78969-72-9
- Methacrylamide
Catalog No.:BCN8157
CAS No.:79-39-0
- Oxytetracycline (Terramycin)
Catalog No.:BCC4819
CAS No.:79-57-2
- Lanosterol
Catalog No.:BCN3332
CAS No.:79-63-0
- Retinyl (Vitamin A) Palmitate
Catalog No.:BCC4749
CAS No.:79-81-2
- Camphene
Catalog No.:BCC9217
CAS No.:79-92-5
- 4'-O-Methylnyasol
Catalog No.:BCN7564
CAS No.:79004-25-4
- Eurycomalin A
Catalog No.:BCN3654
CAS No.:790234-20-7
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
- SNAP
Catalog No.:BCC6712
CAS No.:79032-48-7
- β-Estradiol - d3
Catalog No.:BCC5365
CAS No.:79037-37-9
- L-AP5
Catalog No.:BCC6554
CAS No.:79055-67-7
The role of ionotropic receptors of glutaminic acid in cardiovascular system. A. The influence of ionotropic receptor NMDA agonist - 1R,3R-ACPD and antagonist - DL-AP7 on the systemic pressure in rats.[Pubmed:12768502]
Amino Acids. 2003 Jun;24(4):397-403.
The aim of our study was to estimate the involvement of the peripheral N-methyl-D-aspartate receptors in regulation of cardiovascular function. For this purpose we examined the effects of intravenous injection of the agonists - NMDA (0.025; 0.05 and 1.0 mg/kg iv) and 1R-3R-ACPD (0.025; 0.05 and 1.0 mg/kg iv) - and antagonist of NMDA receptors DL-AP7 (0.02; 0.07 and 0.2 mg/kg iv). To determine if the effects of NMDA come from central or peripheral action we observed the effect during blockade of autonomic ganglion by using the nicotinic receptor antagonist - chlorisondamine (1.25 mg/kg iv). Administration of NMDA in three doses evoked slight hypotension after injection of the medium dose, 0.05 mg/kg. In the condition of pretreatment with 1.25 mg/kg chlorisondamine the hypotensive effect of NMDA was markedly reduced, what might suggest that NMDA-induced hypotension raised from the action within the brain. The competetive NMDA receptor antagonist DL-AP7 slightly increased the blood pressure. None of the injected drug had an influence on the heart rate in our in vivo study. It is concluded that the peripherally localized NMDA receptors may take a part in regulation of cardiovascular system, since their stimulation or blockade evoked the changes of systemic pressure.
The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations.[Pubmed:7042024]
Br J Pharmacol. 1982 Jan;75(1):65-75.
1 The depressant actions on evoked electrical activity and the excitant amino acid antagonist properties of a range of omega-phosphonic alpha-carboxylic amino acids have been investigated in the isolated spinal cord preparations of the frog or immature rat. 2 When tested on dorsal root-evoked ventral root potentials, members of the homologous series from 2- amino-5-phosphonovaleric acid to 2-amino-8-phosphonooctanoic acid showed depressant actions which correlated with the ability of the substances to antagonize selectivity motoneuronal depolarizations induced by N-methyl-D-aspartate. 3 2-Amino-5-phosphonovalerate was the most potent substance of the series giving an apparent KD of 1.4 microM for the antagonism of responses to N-methyl-D-aspartate. 4 A comparison of the (+)- and (-)-forms of 2-amino-5-phosphonovalerate indicated that the N-methyl-D-aspartate antagonist activity and the neuronal depressant action of this substance were both due mainly to the (-)-isomer. 5 The (-)- and (+)-forms of 2-amino-4-phosphonobutyrate had different actions. The (-)-forms of this substance had a relatively weak and non-selective antagonist action on depolarizations induced by N-methyl-D-aspartate, quisqualate and kainate and a similarly weak depressant effect when tested on evoked electrical activity. The (+)-form was more potent than he (-)-form in depressing electrically evoked activity but did not antagonize responses to amino acid excitants. At concentrations higher than those required to depress electrically evoked activity, the (+)-form produced depolarization. This action was blocked by 2-amino-5-phosphonovalerate.


