CampheneCAS# 79-92-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79-92-5 | SDF | Download SDF |
| PubChem ID | 6616 | Appearance | Colorless/white waxy |
| Formula | C10H16 | M.Wt | 136 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol and n-hexane; insoluble in water | ||
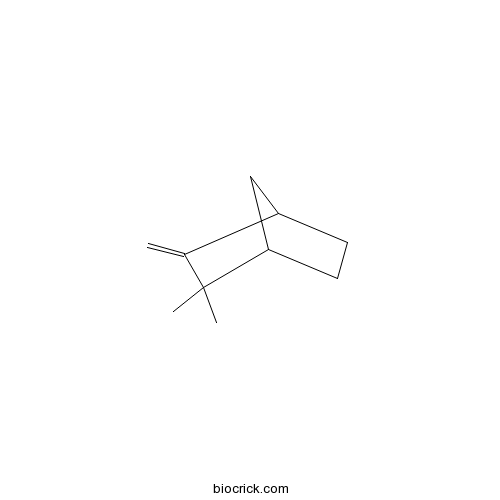
| Chemical Name | 3,3-dimethyl-2-methylidenebicyclo[2.2.1]heptane | ||
| SMILES | CC1(C2CCC(C2)C1=C)C | ||
| Standard InChIKey | CRPUJAZIXJMDBK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16/c1-7-8-4-5-9(6-8)10(7,2)3/h8-9H,1,4-6H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Camphene Dilution Calculator

Camphene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3529 mL | 36.7647 mL | 73.5294 mL | 147.0588 mL | 183.8235 mL |
| 5 mM | 1.4706 mL | 7.3529 mL | 14.7059 mL | 29.4118 mL | 36.7647 mL |
| 10 mM | 0.7353 mL | 3.6765 mL | 7.3529 mL | 14.7059 mL | 18.3824 mL |
| 50 mM | 0.1471 mL | 0.7353 mL | 1.4706 mL | 2.9412 mL | 3.6765 mL |
| 100 mM | 0.0735 mL | 0.3676 mL | 0.7353 mL | 1.4706 mL | 1.8382 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Retinyl (Vitamin A) Palmitate
Catalog No.:BCC4749
CAS No.:79-81-2
- Lanosterol
Catalog No.:BCN3332
CAS No.:79-63-0
- Oxytetracycline (Terramycin)
Catalog No.:BCC4819
CAS No.:79-57-2
- Methacrylamide
Catalog No.:BCN8157
CAS No.:79-39-0
- Guan-fu base G
Catalog No.:BCN8493
CAS No.:78969-72-9
- DL-AP7
Catalog No.:BCC6551
CAS No.:78966-69-5
- L-AP6
Catalog No.:BCC6612
CAS No.:78944-89-5
- Chicanine
Catalog No.:BCN7818
CAS No.:78919-28-5
- Iloprost
Catalog No.:BCC7247
CAS No.:78919-13-8
- Deacetylnimbinene
Catalog No.:BCN4578
CAS No.:912545-53-0
- Orobanone
Catalog No.:BCN3562
CAS No.:78916-35-5
- 6-Thio-dG
Catalog No.:BCC6507
CAS No.:789-61-7
- 4'-O-Methylnyasol
Catalog No.:BCN7564
CAS No.:79004-25-4
- Eurycomalin A
Catalog No.:BCN3654
CAS No.:790234-20-7
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
- SNAP
Catalog No.:BCC6712
CAS No.:79032-48-7
- β-Estradiol - d3
Catalog No.:BCC5365
CAS No.:79037-37-9
- L-AP5
Catalog No.:BCC6554
CAS No.:79055-67-7
- D-AP5
Catalog No.:BCC6553
CAS No.:79055-68-8
- Boc-Alaninol
Catalog No.:BCC2730
CAS No.:79069-13-9
- Boc-Valinol
Catalog No.:BCC2695
CAS No.:79069-14-0
- Boc-Serinol(Bzl)
Catalog No.:BCC2706
CAS No.:79069-15-1
- (-)-Corlumine
Catalog No.:BCN6632
CAS No.:79082-64-7
- Polygalasaponin XXXI
Catalog No.:BCN2857
CAS No.:79103-90-5
Pore Architectures and Mechanical Properties of Porous alpha-SiAlON Ceramics Fabricated via Unidirectional Freeze Casting Based on Camphene-Templating.[Pubmed:30813534]
Materials (Basel). 2019 Feb 26;12(5). pii: ma12050687.
Porous alpha-SiAlON ceramics were fabricated using the Camphene-based unidirectional freeze casting method, in which a gradient porous structure was formed as a result of the decreased solidification velocity in the freezing direction. Microstructure, porosity and pore size distribution of different parts of as-prepared samples were examined and compared, and correlated with their mechanical properties. For a given solid loading, the overall pore size and porosity of the top part were greater than those of the bottom part. Interestingly, despite its higher porosity, the flexural strength and fracture toughness of the top part were both higher than those of the bottom part, suggesting that apart from porosity, pore morphology and size affected mechanical properties of as-prepared porous alpha-SiAlON ceramics.
Properties of oils from plantain pseudostem biotransformed using crude local enzyme sources: a comparison of poultry feed oil.[Pubmed:30556509]
Recent Pat Food Nutr Agric. 2018 Dec 17. pii: FNA-EPUB-95276.
OBJECTIVES: Plantain pseudostem (PPS) wastes were biotransformed by applying simultaneous saccharification and fermentation (SSF) using excised snail digestive juice and yeast slurry, and their oil properties compared with oils from commercially sold poultry feeds (PF). Patents suggesting inclusion of certain additives (US20090226558A1), spices (US5741508A), cysteamine (US4711897A), and dextrin (US6326051B1) in animal diets are regarded as expensive, thus, requiring cheaper and readily available sources of growth. METHODS: The analysis of their free radical scavenging potentials was carried by spectrophotometry, while fatty acids, volatile fatty acids, essentials oils, and phytosterols were determined by chromatography. RESULTS: After biotransformation, the melting point, specific gravity, acid, and peroxide values of the oils from SSF-PPS were significantly lower than those of PF, and showed elevations of C6:0-C18:1(trans-9) fatty acids, palmitic, stearic, gamma-Linolenic, alpha-linolenic, behenic, and lignoceric acids. Camphene, beta-phelandrene, eugenol, beta-elemene, bicyclogermacrene, guaiol, tetradecanoic acid, and hexadecanoic acid levels decreased when PPS was biotransformed. Lactic (1575.75 mg/100g), acetic (1234.26 mg/100g), propionic (845.74 mg/100g), and n-butyric (68.56 mg/100g) acids were the predominant volatile fatty acids (VFAs) in the SSF-PPS oils, which were higher than those found in the PF oil while PF oil contained higher campesterol, Stigmasterol, and 5-avesmasterol. The 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 1,1-diphenyl-2- picrylhydrazyl (DPPH), superoxide, and nitric oxide radical scavenging potentials of PPS oil at high concentrations after biotransformation were equivalent to the standards and the PF oil. CONCLUSION: This study has shown that biotransformation involving snail digestive juice and yeasts extensively improves the oil qualities of agricultural residues sufficient enough for poultry nutrition.


