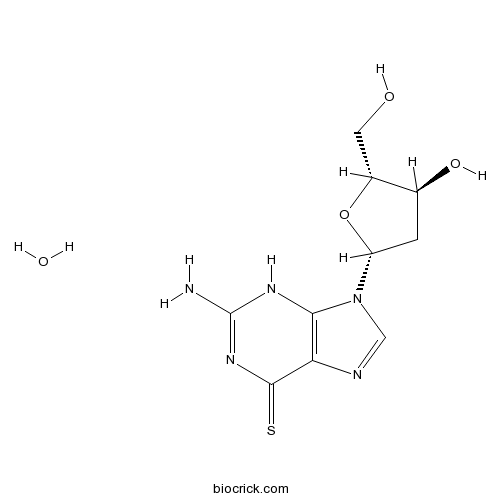
6-Thio-dGtelomere disrupting compound CAS# 789-61-7 |
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 789-61-7 | SDF | Download SDF |
| PubChem ID | 3048840 | Appearance | Powder |
| Formula | C10H13N5O3S | M.Wt | 283.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (352.97 mM; Need ultrasonic) | ||
| Chemical Name | 2-amino-9-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purine-6-thione;hydrate | ||
| SMILES | C1C(C(OC1N2C=NC3=C2NC(=NC3=S)N)CO)O.O | ||
| Standard InChIKey | RAKKINBKYJWQIG-FPKZOZHISA-N | ||
| Standard InChI | InChI=1S/C10H13N5O3S.H2O/c11-10-13-8-7(9(19)14-10)12-3-15(8)6-1-4(17)5(2-16)18-6;/h3-6,16-17H,1-2H2,(H3,11,13,14,19);1H2/t4-,5+,6+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

6-Thio-dG Dilution Calculator

6-Thio-dG Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5297 mL | 17.6485 mL | 35.297 mL | 70.594 mL | 88.2426 mL |
| 5 mM | 0.7059 mL | 3.5297 mL | 7.0594 mL | 14.1188 mL | 17.6485 mL |
| 10 mM | 0.353 mL | 1.7649 mL | 3.5297 mL | 7.0594 mL | 8.8243 mL |
| 50 mM | 0.0706 mL | 0.353 mL | 0.7059 mL | 1.4119 mL | 1.7649 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.353 mL | 0.7059 mL | 0.8824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
6-thio-dG is a nucleoside analogue [1], is a telomerase-mediated telomere disrupting compound [2]. It is an anti-cancer inhibitor [1]. Cancer cells were very sensitive to 6-thio-dG with observed IC50 values ranging from 0.7-2.9 μM, depending on cell types [3].
Telomeres are found at the end of eukaryotic linear chromosomes. They are essential for genomic stability and chromosome maintenance [3].
In HCT116 human colon cancer cell line, treatment with 6-thio-dG made progressive telomere shortening independent of telomerase activity inhibition and induced telomere dysfunction. GRN163L is a telomerase inhibitor. In HCT116 cells, treatment with GRN163L and 6-thio-dG together increased telomere shortening. Within 1 week, 6-thio-dG killed most of HCT116 cells and altered cellular morphology. Normal BJ fibroblast cells are telomerase silent. After 1 week, treatment with 6-thio-dG showed no effect on cell morphology. After long-term treatment with 6-thio-dG, no effect on telomere shortening was found [1].
In murine mode with xenograft derived from A549 lung cancer cell line, as compared to controls, intraperitoneal injection with 2 mg/kg of 6-thio-dG every other day completely prevented progressive tumor growth. Ki67 is a biomarker correlating with proliferation levels. Compared to controls, 6-thio-dG decreased Ki67 staining. Treatment with 6-thio-dG through local injection resulted in even more dramatic decrease in the tumor growth rate compared to untreated controls [3].
References:
[1]. Mender I, Gryaznov S, Dikmen ZG, et al. Abstract LB-125: A novel telomerase inhibitor. Cancer Research, 2013, 73(8 Supplement): LB-125-LB-125.
[2]. Mender I, Gryaznov S, Shay JW. A novel telomerase substrate precursor rapidly induces telomere dysfunction in telomerase positive cancer cells but not telomerase silent normal cells. Oncoscience, 2015, 2(8): 693.
[3]. Mender I, Gryaznov S, Dikmen ZG, et al. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2-deoxyguanosine. Cancer discovery, 2015, 5(1): 82-95.
- 1-chloro-6-(5-ethynylthiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1351
CAS No.:78876-53-6
- 1-chloro-6-(5-(prop-1-ynyl)thiophen-2-yl)hexa-3,5-diyn-2-ol
Catalog No.:BCN1352
CAS No.:78876-52-5
- Demethylasterriquinone B1
Catalog No.:BCC7189
CAS No.:78860-34-1
- Garcinol
Catalog No.:BCC5623
CAS No.:78824-30-3
- Epibrassinolide
Catalog No.:BCC5479
CAS No.:78821-43-9
- 4-Benzyloxycarbonyl-2-piperazinone
Catalog No.:BCC8699
CAS No.:78818-15-2
- Zeylenol
Catalog No.:BCC8267
CAS No.:78804-17-8
- Calcitriol D6
Catalog No.:BCC1447
CAS No.:78782-99-7
- Calcifediol-D6
Catalog No.:BCC4075
CAS No.:78782-98-6
- Deapi-platycodin D
Catalog No.:BCN2614
CAS No.:78763-58-3
- TC 1698 dihydrochloride
Catalog No.:BCC7394
CAS No.:787587-06-8
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
- Orobanone
Catalog No.:BCN3562
CAS No.:78916-35-5
- Deacetylnimbinene
Catalog No.:BCN4578
CAS No.:912545-53-0
- Iloprost
Catalog No.:BCC7247
CAS No.:78919-13-8
- Chicanine
Catalog No.:BCN7818
CAS No.:78919-28-5
- L-AP6
Catalog No.:BCC6612
CAS No.:78944-89-5
- DL-AP7
Catalog No.:BCC6551
CAS No.:78966-69-5
- Guan-fu base G
Catalog No.:BCN8493
CAS No.:78969-72-9
- Methacrylamide
Catalog No.:BCN8157
CAS No.:79-39-0
- Oxytetracycline (Terramycin)
Catalog No.:BCC4819
CAS No.:79-57-2
- Lanosterol
Catalog No.:BCN3332
CAS No.:79-63-0
- Retinyl (Vitamin A) Palmitate
Catalog No.:BCC4749
CAS No.:79-81-2
- Camphene
Catalog No.:BCC9217
CAS No.:79-92-5
Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2'-deoxyguanosine.[Pubmed:25516420]
Cancer Discov. 2015 Jan;5(1):82-95.
UNLABELLED: The relationships between telomerase and telomeres represent attractive targets for new anticancer agents. Here, we report that the nucleoside analogue 6-thio-2'-deoxyguanosine (6-Thio-dG) is recognized by telomerase and is incorporated into de novo-synthesized telomeres. This results in modified telomeres, leading to telomere dysfunction, but only in cells expressing telomerase. 6-Thio-dG, but not 6-thioguanine, induced telomere dysfunction in telomerase-positive human cancer cells and hTERT-expressing human fibroblasts, but not in telomerase-negative cells. Treatment with 6-Thio-dG resulted in rapid cell death for the vast majority of the cancer cell lines tested, whereas normal human fibroblasts and human colonic epithelial cells were largely unaffected. In A549 lung cancer cell-based mouse xenograft studies, 6-Thio-dG caused a decrease in the tumor growth rate superior to that observed with 6-thioguanine treatment. In addition, 6-Thio-dG increased telomere dysfunction in tumor cells in vivo. These results indicate that 6-Thio-dG may provide a new telomere-addressed telomerase-dependent anticancer approach. SIGNIFICANCE: Telomerase is an almost universal oncology target, yet there are few telomerase-directed therapies in human clinical trials. In the present study, we demonstrate a small-molecule telomerase substrate approach that induces telomerase-mediated targeted "telomere uncapping," but only in telomerase-positive cancer cells, with minimal effects in normal telomerase-negative cells.
Agonists of Toll-like receptor 9 containing synthetic dinucleotide motifs.[Pubmed:17988082]
J Med Chem. 2007 Dec 13;50(25):6411-8.
Oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs activate Toll-like receptor 9 (TLR9). Our previous studies have shown that ODNs containing two 5'-ends are more immunostimulatory than those with one 5'-end. In the present study, to understand the role of functional groups in TLR9 recognition and subsequent immune response, we substituted C or G of a CpG dinucleotide with 5-OH-dC, 5-propyne-dC, furano-dT, 1-(2'-deoxy-beta- d-ribofuranosyl)-2-oxo-7-deaza-8-methyl-purine, dF, 4-thio-dU, N(3)-Me-dC, N (4)-Et-dC, Psi-iso-dC, and arabinoC or 7-deaza-dG, 7-deaza-8-aza-dG, 9-deaza-dG, N(1)-Me-dG, N(2)-Me-dG, 6-Thio-dG, dI, 8-OMe-dG, 8-O-allyl-dG, and arabinoG in ODN containing two 5'-ends. Agonists of TLR9 containing cytosine or guanine modification showed activity in HEK293 cells expressing TLR9, mouse spleen, and human cell-based assays and in vivo in mice. The results presented here provide insight into which specific chemical modifications at C or G of the CpG motif are recognized by TLR9 and the ability to modulate immune responses substituting natural C or G in immune modulatory oligonucleotides.


