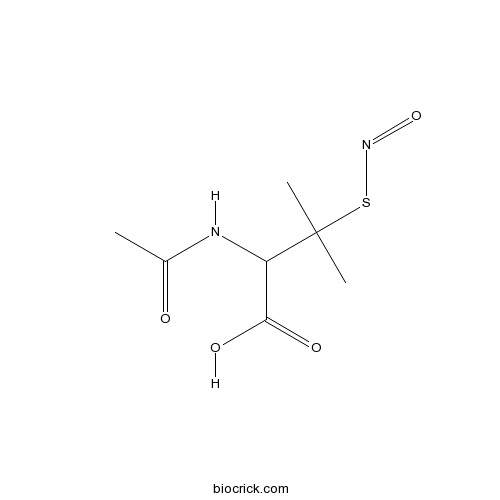
SNAPA stable analog of endogenous S-nitroso compounds CAS# 79032-48-7 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79032-48-7 | SDF | Download SDF |
| PubChem ID | 5231 | Appearance | Powder |
| Formula | C7H12N2O4S | M.Wt | 220.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | 2-acetamido-3-methyl-3-nitrososulfanylbutanoic acid | ||
| SMILES | CC(=O)NC(C(=O)O)C(C)(C)SN=O | ||
| Standard InChIKey | ZIIQCSMRQKCOCT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H12N2O4S/c1-4(10)8-5(6(11)12)7(2,3)14-9-13/h5H,1-3H3,(H,8,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A stable analog of endogenous S-nitroso compounds. A source of NO in vivo which unlike organic O-nitrates does not induce tolerance. Decomposes slowly in solution with a t½ of 37h. |

SNAP Dilution Calculator

SNAP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5405 mL | 22.7025 mL | 45.405 mL | 90.81 mL | 113.5125 mL |
| 5 mM | 0.9081 mL | 4.5405 mL | 9.081 mL | 18.162 mL | 22.7025 mL |
| 10 mM | 0.4541 mL | 2.2703 mL | 4.5405 mL | 9.081 mL | 11.3513 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9081 mL | 1.8162 mL | 2.2703 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9081 mL | 1.1351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
- Eurycomalin A
Catalog No.:BCN3654
CAS No.:790234-20-7
- 4'-O-Methylnyasol
Catalog No.:BCN7564
CAS No.:79004-25-4
- Camphene
Catalog No.:BCC9217
CAS No.:79-92-5
- Retinyl (Vitamin A) Palmitate
Catalog No.:BCC4749
CAS No.:79-81-2
- Lanosterol
Catalog No.:BCN3332
CAS No.:79-63-0
- Oxytetracycline (Terramycin)
Catalog No.:BCC4819
CAS No.:79-57-2
- Methacrylamide
Catalog No.:BCN8157
CAS No.:79-39-0
- Guan-fu base G
Catalog No.:BCN8493
CAS No.:78969-72-9
- DL-AP7
Catalog No.:BCC6551
CAS No.:78966-69-5
- L-AP6
Catalog No.:BCC6612
CAS No.:78944-89-5
- Chicanine
Catalog No.:BCN7818
CAS No.:78919-28-5
- β-Estradiol - d3
Catalog No.:BCC5365
CAS No.:79037-37-9
- L-AP5
Catalog No.:BCC6554
CAS No.:79055-67-7
- D-AP5
Catalog No.:BCC6553
CAS No.:79055-68-8
- Boc-Alaninol
Catalog No.:BCC2730
CAS No.:79069-13-9
- Boc-Valinol
Catalog No.:BCC2695
CAS No.:79069-14-0
- Boc-Serinol(Bzl)
Catalog No.:BCC2706
CAS No.:79069-15-1
- (-)-Corlumine
Catalog No.:BCN6632
CAS No.:79082-64-7
- Polygalasaponin XXXI
Catalog No.:BCN2857
CAS No.:79103-90-5
- Lariciresinol acetate
Catalog No.:BCN4577
CAS No.:79114-77-5
- Hannokinol
Catalog No.:BCN6514
CAS No.:79120-40-4
- A-7 hydrochloride
Catalog No.:BCC6625
CAS No.:79127-24-5
- Diammonium glycyrrhizinate
Catalog No.:BCN7145
CAS No.:79165-06-3
SNAP: A General Purpose Network Analysis and Graph Mining Library.[Pubmed:28344853]
ACM Trans Intell Syst Technol. 2016 Oct;8(1).
Large networks are becoming a widely used abstraction for studying complex systems in a broad set of disciplines, ranging from social network analysis to molecular biology and neuroscience. Despite an increasing need to analyze and manipulate large networks, only a limited number of tools are available for this task. Here, we describe Stanford Network Analysis Platform (SNAP), a general-purpose, high-performance system that provides easy to use, high-level operations for analysis and manipulation of large networks. We present SNAP functionality, describe its implementational details, and give performance benchmarks. SNAP has been developed for single big-memory machines and it balances the trade-off between maximum performance, compact in-memory graph representation, and the ability to handle dynamic graphs where nodes and edges are being added or removed over time. SNAP can process massive networks with hundreds of millions of nodes and billions of edges. SNAP offers over 140 different graph algorithms that can efficiently manipulate large graphs, calculate structural properties, generate regular and random graphs, and handle attributes and meta-data on nodes and edges. Besides being able to handle large graphs, an additional strength of SNAP is that networks and their attributes are fully dynamic, they can be modified during the computation at low cost. SNAP is provided as an open source library in C++ as well as a module in Python. We also describe the Stanford Large Network Dataset, a set of social and information real-world networks and datasets, which we make publicly available. The collection is a complementary resource to our SNAP software and is widely used for development and benchmarking of graph analytics algorithms.
Early Golgi Abnormalities and Neurodegeneration upon Loss of Presynaptic Proteins Munc18-1, Syntaxin-1, or SNAP-25.[Pubmed:28348137]
J Neurosci. 2017 Apr 26;37(17):4525-4539.
The loss of presynaptic proteins Munc18-1, syntaxin-1, or SNAP-25 is known to produce cell death, but the underlying features have not been compared experimentally. Here, we investigated these features in cultured mouse CNS and DRG neurons. Side-by-side comparisons confirmed massive cell death, before synaptogenesis, within 1-4 DIV upon loss of t-SNAREs (syntaxin-1, SNAP-25) or Munc18-1, but not v-SNAREs (synaptobrevins/VAMP1/2/3 using tetanus neurotoxin (TeNT), also in TI-VAMP/VAMP7 knock-out (KO) neurons). A condensed cis-Golgi was the first abnormality observed upon Munc18-1 or SNAP-25 loss within 3 DIV. This phenotype was distinct from the Golgi fragmentation observed in apoptosis. Cell death was too rapid after syntaxin-1 loss to study Golgi abnormalities. Syntaxin-1 and Munc18-1 depend on each other for normal cellular levels. We observed that endogenous syntaxin-1 accumulates at the Golgi of Munc18-1 KO neurons. However, expression of a non-neuronal Munc18 isoform that does not bind syntaxin-1, Munc18-3, in Munc18-1 KO neurons prevented cell death and restored normal cis-Golgi morphology, but not synaptic transmission or syntaxin-1 targeting. Finally, we observed that DRG neurons are the only Munc18-1 KO neurons that do not degenerate in vivo or in vitro In these neurons, cis-Golgi abnormalities were less severe, with no changes in Golgi shape. Together, these data demonstrate that cell death upon Munc18-1, syntaxin-1, or SNAP-25 loss occurs via a degenerative pathway unrelated to the known synapse function of these proteins and involving early cis-Golgi abnormalities, distinct from apoptosis.SIGNIFICANCE STATEMENT This study provides new insights in a neurodegeneration pathway triggered by the absence of specific proteins involved in synaptic transmission (syntaxin-1, Munc18-1, SNAP-25), whereas other proteins involved in the same molecular process (synaptobrevins, Munc13-1/2) do not cause degeneration. Massive cell death occurs in cultured neurons upon depleting syntaxin-1, Munc18-1, and/or SNAP-25, well before synapse formation. This study characterizes several relevant cellular phenotypes, especially early cis-Golgi abnormalities, distinct from abnormalities observed during apoptosis, and rules out several other phenotypes as causal (defects in syntaxin-1 targeting and synaptic transmission). As proteins, such as syntaxin-1, Munc18-1, or SNAP-25, modulate alpha-synuclein neuropathy and/or are dysregulated in Alzheimer's disease, understanding this type of neurodegeneration may provide new links between synaptic defects and neurodegeneration in humans.
A novel therapeutic with two SNAP-25 inactivating proteases shows long-lasting anti-hyperalgesic activity in a rat model of neuropathic pain.[Pubmed:28347837]
Neuropharmacology. 2017 May 15;118:223-232.
A pressing need exists for long-acting, non-addictive medicines to treat chronic pain, a major societal burden. Botulinum neurotoxin type A (BoNT/A) complex - a potent, specific and prolonged inhibitor of neuro-exocytosis - gives some relief in several pain disorders, but not for all patients. Our study objective was to modify BoNT/A to overcome its inability to block transmitter release elicited by high [Ca(2+)]i and increase its limited analgesic effects. This was achieved by fusing a BoNT/A gene to that for the light chain (LC) of type/E. The resultant purified protein, LC/E-BoNT/A, entered cultured sensory neurons and, unlike BoNT/A, inhibited release of calcitonin gene-related peptide evoked by capsaicin. Western blotting revealed that this improvement could be due to a more extensive truncation by LC/E of synaptosomal-associated protein of Mr = 25 k, essential for neuro-exocytosis. When tested in a rat spared nerve injury (SNI) model, a single intra-plantar (IPL) injection of LC/E-BoNT/A alleviated for approximately 2 weeks mechanical and cold hyper-sensitivities, in a dose-dependent manner. The highest non-paralytic dose (75 U/Kg, IPL) proved significantly more efficacious than BoNT/A (15 U/Kg, IPL) or repeated systemic pregabalin (10 mg/Kg, intraperitoneal), a clinically-used pain modulator. Effects of repeated or delayed injections of this fusion protein highlighted its analgesic potential. Attenuation of mechanical hyperalgesia was extended by a second administration when the effect of the first had diminished. When injected 5 weeks after injury, LC/E-BoNT/A also reversed fully-established mechanical and cold hyper-sensitivity. Thus, combining advantageous features of BoNT/E and/A yields an efficacious, locally-applied and long-acting anti-hyperalgesic.
Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production.[Pubmed:10401570]
Br J Pharmacol. 1999 Jun;127(3):779-87.
1. Sodium nitroprusside, S-nitroso-N-acetyl-D,L-penicillamine, Spermine NONOate and DEA NONOate raised cyclic GMP levels in bovine chromaffin cells in a time and concentration dependent manner with different potencies, the most potent being DEA/NO with an EC50 value of 0.38 +/- 0.02 microM. 2. Measurements of NO released from these donors revealed that DEA/NO decomposed with a half-life (t1/2) of 3.9 +/- 0.2 min. The t1/2 for SPER/NO was 37 +/- 3 min. SNAP decomposed more slowly (t1/2 = 37 +/- 4 h) and after 60 min the amount of NO produced corresponded to less than 2% of the total SNAP present. The rate of NO production from SNAP was increased by the presence of glutathione. 3. For DEA/NO and SPER/NO there was a clear correlation between nitric oxide production and cyclic GMP increases. Their threshold concentrations were 0.05 microM and maximal effective concentration between 2.5 and 5 microM. 4. For SNAP, threshold activation was seen at 1 microM, whereas full activation required a higher concentration (500-750 microM). The dose-response for SNAP increases in cyclic GMP was shifted nearly two orders of magnitude lower in the presence of glutathione. At higher concentrations an inhibition of cyclic GMP accumulation was found. This effect was not observed with either the nitric oxide-deficient SNAP analogue or other NO donors. 5. Although NO-donors are likely to be valuable for studying NO functions, their effective concentrations and the amount of NO released by them are very different and should be assessed in each system to ensure that physiological concentrations of NO are used.
Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: modulation by pituitary adenylate cyclase-activating peptide, forskolin and guanylate cyclase inhibitors.[Pubmed:9336304]
J Pharmacol Exp Ther. 1997 Oct;283(1):23-8.
This study examines nitric oxide (NO) mediated effects on longitudinal muscle with adherent myenteric ganglia from rat ileum in vitro using NO donors and electrical field stimulation. Electrical field stimulation (20 Hz) caused a biphasic response-a relaxation followed by a contraction. NG-nitro-L-arginine methyl ester almost totally abolished the relaxation and L-arginine restored it. The contraction was unaffected. The NO donors sodium-nitroso-N-acetylpenicillamine (SNAP) and sodium-nitroprusside also induced a biphasic response, a contraction followed by relaxation. Relaxations mediated by neuronally released NO were not blocked by methylene blue or 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one suggesting that they are independent of a rise in intracellular cyclic guanylate cyclase. Their amplitude was unaffected by forskolin. The relaxations evoked by NO (or a NO-related substance) liberated from SNAP were blocked by methylene blue or 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one indicating a cyclic guanylate cyclase-dependent mechanism of action. Pituitary adenylate cyclase-activating peptide and forskolin, but not vasoactive intestinal peptide or neuropeptide Y, caused a marked leftward shift of the concentration-response curve of the SNAP-induced relaxation. The contractions induced by SNAP were blocked by methylene blue and 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one and thus, cyclic guanylate cyclase dependent. The SNAP-induced contractions were abolished by pituitary adenylate cyclase-activating peptide and forskolin, but unaffected by vasoactive intestinal peptide or NPY. In conclusion, motor responses evoked by NO released from NO donors vs. neuronally released NO reveals different mechanisms of action.
Neurotoxicity in conscious rats following intraventricular SNAP, a nitric oxide donor.[Pubmed:7969812]
Neuropharmacology. 1994 Jul;33(7):915-27.
A solution containing S-nitroso-N-acetylpenicillamine (SNAP), a nitric oxide (NO.-releasing compound, was microinjected in doses of 0.25-2 mumol into a lateral ventricle of conscious rats. SNAP produced dose-dependent convulsions similar to those associated with limbic stimulation, such as tonic extension of the hindlimbs and tail, and dystonia of the forepaws. At 2 mumol, SNAP evoked hyperventilation (arterial hypocapnia), arterial hyperglycemia and caused necrotic lesions of periventricular gray (e.g. lateral septal nucleus) and white matter structures. In the caudate nucleus and lateral septal nucleus ipsilateral to injection, SNAP elicited a bipolar metabolic pattern of low glucose metabolism proximal to the ventricle with higher values occurring more distally. In control studies, we proved that the residue of SNAP decomposition, N-acetylpenicillamine disulfide injected intraventricularly (2 mumol), was without physiological, behavioral, or histological effects. Ventricular pretreatment with methylene blue (2 nmol), a putative inhibitor of guanylate cyclase and superoxide generator, suppressed several of the behavioral manifestations of 1 mumol SNAP, such as the forepaw dystonia, squinting, and facial clonus, but was ineffective on the physiological and histological variables affected by the 2 mumol SNAP dose. Another NO. donor, sodium nitroprusside (2 mumol), produced fewer behavioral and cytotoxic effects over a 55-min observation period, but caused more intense and widely distributed metabolic stimulation, especially in commissural and projection white matter tracts. The results are the basis for a conscious rat model using intraventricular injection of nitrocompounds to examine the physiological, behavioral, metabolic and cytotoxic properties of NO. in the brain.
Differential hemodynamic effects and tolerance properties of nitroglycerin and an S-nitrosothiol in experimental heart failure.[Pubmed:1899118]
J Pharmacol Exp Ther. 1991 Jan;256(1):249-54.
S-nitrosothiols are potent in vitro vasodilators, but little is known about their in vivo action. In this study, we compared the effects of S-nitroso N-acetyl penicillamine (SNAP) and nitroglycerin (NTG) on left ventricular (LV) hemodynamics in congestive heart failure rats. By using a twoday crossover design, stepwise i.v. infusions of SNAP or NTG at 3, 5 and 8 micrograms/min were administered for 30 min each, followed by a dose of 10 micrograms/min over the next 10 h. LV end-diastolic and peak-systolic pressures (LVEDP and LVPSP, respectively) were measured at selected intervals. SNAP and NTG produced maximal LVEDP reductions of 46 and 44%, respectively, at the highest infusion rate. However, at the lower doses, greater reductions of LVEDP were seen with SNAP. NTG had a smaller effect on LVPSP (maximum 6% reduction) than SNAP (maximum reduction of 15%). During the 10-h infusion of NTG, LVEDP gradually returned to base-line values, indicating the development of tolerance, despite relatively constant plasma levels of NTG over the infusion period. Tolerance in LVEDP effects was not observed during the 10-h infusion of SNAP. In the presence of NTG tolerance, rats were still responsive to SNAP (mean reduction of LVEDP 24%), suggesting the absence of cross-tolerance between these two nitrovasodilators. These results suggest that SNAP is a more potent in vivo vasodilator than NTG, has more arterial action than NTG and is less prone to produce LV hemodynamic tolerance.


