NSC 95397Selective Cdc25 dual specificity phosphatase inhibitor CAS# 93718-83-3 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 93718-83-3 | SDF | Download SDF |
| PubChem ID | 262093 | Appearance | Powder |
| Formula | C14H14O4S2 | M.Wt | 310.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (322.18 mM) *"≥" means soluble, but saturation unknown. | ||
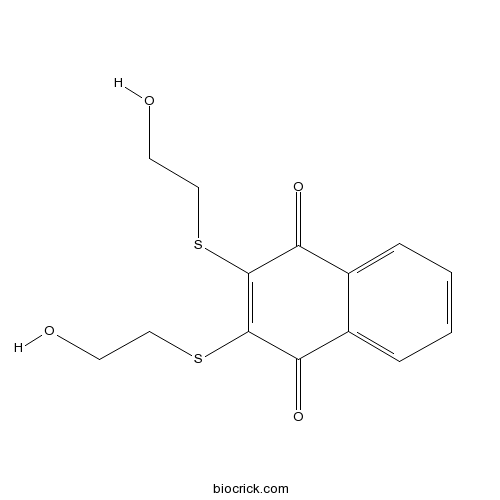
| Chemical Name | 2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=C(C2=O)SCCO)SCCO | ||
| Standard InChIKey | MAASHDQFQDDECQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective irreversible inhibitor of Cdc25 dual specificity phosphatases (Ki values are 32, 96 and 40 nM for inhibition of Cdc25A, -B and -C respectively). Displays 125 - 180-fold selectivity over VH1-related dual-specificity phosphatase and protein tyrosine phosphatase 1b. Inhibits carcinoma cell growth and blocks G2/M phase transition in vitro. |

NSC 95397 Dilution Calculator

NSC 95397 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2219 mL | 16.1093 mL | 32.2186 mL | 64.4371 mL | 80.5464 mL |
| 5 mM | 0.6444 mL | 3.2219 mL | 6.4437 mL | 12.8874 mL | 16.1093 mL |
| 10 mM | 0.3222 mL | 1.6109 mL | 3.2219 mL | 6.4437 mL | 8.0546 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2887 mL | 1.6109 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6444 mL | 0.8055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GSK690693
Catalog No.:BCC2483
CAS No.:937174-76-0
- Leuconolam
Catalog No.:BCN4482
CAS No.:93710-27-1
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- Magnolignan C
Catalog No.:BCN4085
CAS No.:93697-42-8
- OSI-027
Catalog No.:BCC4603
CAS No.:936890-98-1
- Forsythoside E
Catalog No.:BCN2782
CAS No.:93675-88-8
- Rengyol
Catalog No.:BCN4481
CAS No.:93675-85-5
- Magnolignan A
Catalog No.:BCN4084
CAS No.:93673-81-5
- VX-809
Catalog No.:BCC3712
CAS No.:936727-05-8
- LCZ696
Catalog No.:BCC5505
CAS No.:936623-90-4
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
- PCI-32765 Racemate
Catalog No.:BCC5124
CAS No.:936563-87-0
- ARRY-380
Catalog No.:BCC3726
CAS No.:937265-83-3
- SB1317
Catalog No.:BCC1925
CAS No.:937270-47-8
- Pacritinib (SB1518)
Catalog No.:BCC4558
CAS No.:937272-79-2
- Magnaldehyde D
Catalog No.:BCN4070
CAS No.:93753-33-4
- GRP (human)
Catalog No.:BCC5810
CAS No.:93755-85-2
- Jangomolide
Catalog No.:BCN4483
CAS No.:93767-25-0
- Neogambogic acid
Catalog No.:BCN2321
CAS No.:93772-31-7
- Neurodazine
Catalog No.:BCC7738
CAS No.:937807-66-4
- gamma-Secretase Modulators
Catalog No.:BCC1586
CAS No.:937812-80-1
- Roxatidine Acetate HCl
Catalog No.:BCC4534
CAS No.:93793-83-0
- 3-Prenyl-2,4,6-trihydroxybenzophenone
Catalog No.:BCN1303
CAS No.:93796-20-4
- 22-beta-Acetoxyglycyrrhizin
Catalog No.:BCN7904
CAS No.:938042-17-2
Stimulation of Suicidal Erythrocyte Death by the CDC25 Inhibitor NSC-95397.[Pubmed:27889774]
Cell Physiol Biochem. 2016;40(3-4):597-607.
BACKGROUND/AIMS: The CDC25B inhibitor NSC-95397 triggers apoptosis of tumor cells and is thus considered for the treatment of malignancy. The substance is effective in part by modification of gene expression. Similar to apoptosis of nucleated cells erythrocytes may undergo eryptosis, the suicidal erythrocyte death characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the erythrocyte surface. Eryptosis may be triggered by increase of cytosolic Ca2+ activity ([Ca2+]i), oxidative stress, ceramide, as well as activation of protein kinases. The present study explored, whether NSC-95397 induces eryptosis and, if so, to shed some light on the mechanisms involved. METHODS: Phosphatidylserine exposure at the cell surface was estimated from annexin-V-binding, cell volume from forward scatter, [Ca2+]i from Fluo3-fluorescence, ROS formation from DCFDA dependent fluorescence, and ceramide abundance utilizing specific antibodies. RESULTS: A 48 hours exposure of human erythrocytes to NSC-95397 significantly increased the percentage of annexin-V-binding cells (>/= 1 microM), significantly decreased forward scatter (>/= 2.5 microM), and significantly increased Fluo3-fluorescence (>/= 1 microM), DCFDA fluorescence (5 microM) and ceramide abundance (>/= 5 microM). The effect of NSC-95397 (5 microM) on annexin-V-binding was slightly, but significantly blunted by removal of extracellular Ca2+ and by addition of the protein kinase C inhibitor staurosporine (1 microM). CONCLUSIONS: NSC-95397 triggers cell shrinkage and phospholipid scrambling of the erythrocyte cell membrane, an effect in part requiring entry of Ca2+ and activation of staurosporine sensitive kinase(s).
The cytotoxic agents NSC-95397, brefeldin A, bortezomib and sanguinarine induce apoptosis in neuroendocrine tumors in vitro.[Pubmed:20150630]
Anticancer Res. 2010 Jan;30(1):149-56.
UNLABELLED: The aim of this study was to investigate the apoptosis resulting from NSC 95397, brefeldin A, bortezomib and sanguinarine in neuroendocrine tumor cell lines. MATERIALS AND METHODS: A multiparametric high-content screening assay for measurement of apoptosis was used. The human pancreatic carcinoid cell line, BON-1, human typical bronchial carcinoid cell line NCI-H727 and the human atypical bronchial carcinoid cell line NCI-H720 were tested. After incubation with cytotoxic drugs, the DNA-binding dye Hoechst 33342, fluorescein-tagged probes that covalently bind active caspase-3 and chloromethyl-X-rosamine to detect mitochondrial membrane potential were added. Image acquisition and quantitative measurement of fluorescence was performed using automated image capture and analysis instrument ArrayScan. In addition, nuclear morphology was examined on microscopic slides stained with May-Grunewald-Giemsa. RESULTS: A time- and dose-dependent activation of caspase-3 and increase in nuclear fragmentation and condensation were observed for the drugs using a multiparametric apoptosis assay. These results were confirmed with nuclear morphological examination on microscopic slides. CONCLUSION: NSC 95397, brefeldin A, bortezomib and sanguinarine induced caspase-3 activation with modest changes in nuclear morphology.
Quinone-induced Cdc25A inhibition causes ERK-dependent connexin phosphorylation.[Pubmed:15652497]
Biochem Biophys Res Commun. 2005 Feb 25;327(4):1016-23.
Gap junctional intercellular communication (GJC) varies during progression of the cell cycle. We propose here that Cdc25A, a dual specificity phosphatase crucial for cell cycle progression, is linked to connexin (Cx) phosphorylation and the modulation of GJC. Inhibition of Cdc25 phosphatases in rat liver epithelial cells employing a 1,4-naphthoquinone-based inhibitor, NSC95397, induced cell cycle arrest, tyrosine phosphorylation of the epidermal growth factor receptor (EGFR), and activation of extracellular signal-regulated kinases ERK-1 and -2. ERK activation was blocked by specific inhibitors of MAPK/ERK kinases 1/2 or of the EGFR tyrosine kinase. An EGFR-dephosphorylation assay suggested that Cdc25A interacts with the EGFR, with inhibition by NSC95397 resulting in activation of the receptor. As a consequence of ERK activation, Cx43 was phosphorylated, resulting in a downregulation of GJC. Loss of GJC was prevented by inhibition of ERK activation. In summary, cell cycle and GJC are connected via Cdc25A and the EGFR-ERK pathway.
NAD(P)H:quinone oxidoreductase-1-dependent and -independent cytotoxicity of potent quinone Cdc25 phosphatase inhibitors.[Pubmed:14718602]
J Pharmacol Exp Ther. 2004 Apr;309(1):64-70.
Cdc25 dual-specificity phosphatases coordinate cell cycle progression and cellular signaling. Consequently, Cdc25 inhibitors represent potential anticancer agents. We evaluated >10,000 compounds for inhibition of human Cdc25 phosphatases and identified many potent and selective inhibitors, which all contained a quinone. Bioreductive enzymes frequently detoxify or activate quinones. Therefore, we evaluated the effect of NAD(P)H:quinone oxidoreductase-1 (NQO1) and reductase-rich microsomes on the activity of three quinone-containing Cdc25 inhibitors: 2-(2-hydroxyethylsulfanyl)-3-methyl-1,4-naphthoquinone (Cpd 5, compound 5; NSC 672121), 2,3-bis-(2-hydroxyethylsulfanyl)-1,4-naphthoquinone (NSC 95397), and 6-chloro-7-(2-morpholin-4-yl-ethylamino)quinoline-5,8-dione (NSC 663284). Each inhibitor was reduced by human NQO1 (K(m) of 0.3-0.5 microM) but none by microsomes. Compounds were evaluated with six cancer cell lines containing different amounts of NQO1: HT-29 (1056 nmol/mg/min), HCT116 (660 nmol/mg/min), sublines HCT116-R30A (28 nmol/mg/min) and HCT-116R30A/NQ5 (934 nmol/mg/min), MDA-MB-231/Q2 (null NQO1), and subline MDA-MB-231/Q6 (124 nmol/mg/min) but containing similar amounts of microsomal cytochrome P450 reductase and cytochrome b(5) reductase. Growth inhibition and G2/M arrest by Cpd 5 was proportional to NQO1 levels, requiring 4- to 5-fold more Cpd 5 to inhibit HCT-116 or HCT-116R30A/NQ5 compared with HCT-116R30A. In contrast, in all tested cell lines irrespective of NQO1 level, growth inhibition and G2/M arrest by NSC 95375 and NSC 663284 were similar (average IC(50) of 1.3 +/- 0.3 and 2.6 +/- 0.4 microM, respectively). NSC 95375 and NSC 663284 also caused similar Cdk1 hyperphosphorylation, indicating similar Cdc25 inhibition. However, lower Cpd 5 concentrations were needed to produce Cdk1 hyperphosphorylation in sublines with minimal NQO1. Thus, NQO1 detoxified Cpd 5, probably by reducing it to a less active hydroquinone, whereas NSC 95397- and NSC 663284-generated cytotoxicity was unaffected by NQO1.


