NeurodazineInduces neurogenesis in mature skeletal muscle cells CAS# 937807-66-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 937807-66-4 | SDF | Download SDF |
| PubChem ID | 16112820 | Appearance | Powder |
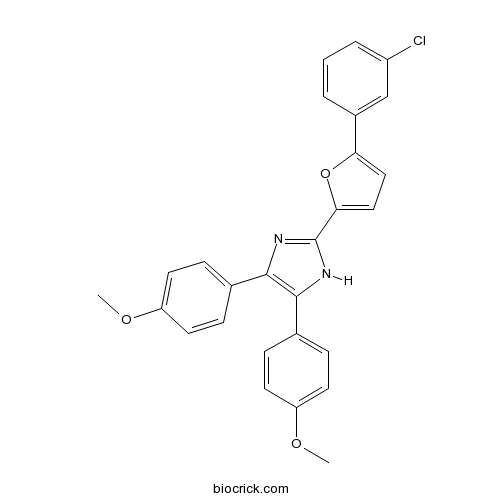
| Formula | C27H21ClN2O3 | M.Wt | 456.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 2-[5-(3-chlorophenyl)furan-2-yl]-4,5-bis(4-methoxyphenyl)-1H-imidazole | ||
| SMILES | COC1=CC=C(C=C1)C2=C(N=C(N2)C3=CC=C(O3)C4=CC(=CC=C4)Cl)C5=CC=C(C=C5)OC | ||
| Standard InChIKey | FEEOFPAEDSMOTO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H21ClN2O3/c1-31-21-10-6-17(7-11-21)25-26(18-8-12-22(32-2)13-9-18)30-27(29-25)24-15-14-23(33-24)19-4-3-5-20(28)16-19/h3-16H,1-2H3,(H,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Induces neurogenesis of non-pluripotent C2C12 myoblasts and cells derived from mature human skeletal muscle. Upregulates the expression of neuron-specific markers in C2C12 cells. |

Neurodazine Dilution Calculator

Neurodazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1886 mL | 10.9428 mL | 21.8857 mL | 43.7713 mL | 54.7142 mL |
| 5 mM | 0.4377 mL | 2.1886 mL | 4.3771 mL | 8.7543 mL | 10.9428 mL |
| 10 mM | 0.2189 mL | 1.0943 mL | 2.1886 mL | 4.3771 mL | 5.4714 mL |
| 50 mM | 0.0438 mL | 0.2189 mL | 0.4377 mL | 0.8754 mL | 1.0943 mL |
| 100 mM | 0.0219 mL | 0.1094 mL | 0.2189 mL | 0.4377 mL | 0.5471 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neogambogic acid
Catalog No.:BCN2321
CAS No.:93772-31-7
- Jangomolide
Catalog No.:BCN4483
CAS No.:93767-25-0
- GRP (human)
Catalog No.:BCC5810
CAS No.:93755-85-2
- Magnaldehyde D
Catalog No.:BCN4070
CAS No.:93753-33-4
- Pacritinib (SB1518)
Catalog No.:BCC4558
CAS No.:937272-79-2
- SB1317
Catalog No.:BCC1925
CAS No.:937270-47-8
- ARRY-380
Catalog No.:BCC3726
CAS No.:937265-83-3
- NSC 95397
Catalog No.:BCC7109
CAS No.:93718-83-3
- GSK690693
Catalog No.:BCC2483
CAS No.:937174-76-0
- Leuconolam
Catalog No.:BCN4482
CAS No.:93710-27-1
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- Magnolignan C
Catalog No.:BCN4085
CAS No.:93697-42-8
- gamma-Secretase Modulators
Catalog No.:BCC1586
CAS No.:937812-80-1
- Roxatidine Acetate HCl
Catalog No.:BCC4534
CAS No.:93793-83-0
- 3-Prenyl-2,4,6-trihydroxybenzophenone
Catalog No.:BCN1303
CAS No.:93796-20-4
- 22-beta-Acetoxyglycyrrhizin
Catalog No.:BCN7904
CAS No.:938042-17-2
- ATPγS tetralithium salt
Catalog No.:BCC7855
CAS No.:93839-89-5
- KU-0063794
Catalog No.:BCC2484
CAS No.:938440-64-3
- Isochamaejasmine
Catalog No.:BCN3128
CAS No.:93859-63-3
- 9-Oxo-2,7-bisaboladien-15-oic acid
Catalog No.:BCN4484
CAS No.:93888-59-6
- FIPI
Catalog No.:BCC7721
CAS No.:939055-18-2
- Hirsutanonol 5-O-glucoside
Catalog No.:BCN4485
CAS No.:93915-36-7
- Toonaciliatin M
Catalog No.:BCN7881
CAS No.:93930-04-2
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
Triorganoindium reagents in selective palladium-catalyzed cross-coupling with iodoimidazoles: synthesis of neurodazine.[Pubmed:25203769]
J Org Chem. 2014 Oct 17;79(20):9586-93.
Triorganoindium reagents (R3In, R = aryl, heteroaryl, alkynyl) react selectively under palladium catalysis with N-benzyl-2,4,5-triiodoimidazole to afford the C-2 monocoupling products. The reaction proceeds efficiently for a variety of aryl- and heteroarylindium reagents with the transfer of all three organic groups attached to the metal. The coupling products can be used in a subsequent two-fold cross-coupling to give trisubstituted imidazoles in good yields. This approach was employed to synthesize Neurodazine and analogues in good yields.
Combining Suppression of Stemness with Lineage-Specific Induction Leads to Conversion of Pluripotent Cells into Functional Neurons.[Pubmed:26590637]
Chem Biol. 2015 Nov 19;22(11):1512-1520.
Sox2 is a key player in the maintenance of pluripotency and stemness, and thus inhibition of its function would abrogate the stemness of pluripotent cells and induce differentiation into several types of cells. Herein we describe a strategy that relies on a combination of Sox2 inhibition with lineage-specific induction to promote efficient and selective differentiation of pluripotent P19 cells into neurons. When P19 cells transduced with Skp protein, an inhibitor of Sox2, are incubated with a neurogenesis inducer, the cells are selectively converted into neurons that generate depolarization-induced sodium currents and action potentials. This finding indicates that the differentiated neurons are electrophysiologically active. Signaling pathway studies lead us to conclude that a combination of Skp with the neurogenesis inducer enhances neurogenesis in P19 cells by activating Wnt and Notch pathways. The present differentiation protocol could be valuable to selectively generate functionally active neurons from pluripotent cells.


