gamma-Secretase ModulatorsAmyloid-β production inhibitor CAS# 937812-80-1 |
- DMXAA (Vadimezan)
Catalog No.:BCC3644
CAS No.:117570-53-3
- YH239-EE
Catalog No.:BCC5454
CAS No.:1364488-67-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 937812-80-1 | SDF | Download SDF |
| PubChem ID | 24781184 | Appearance | Powder |
| Formula | C26H24F3N3O3 | M.Wt | 483.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Amyloid-β production inhibitor; γ-Secretase Modulators | ||
| Solubility | Soluble in DMSO | ||
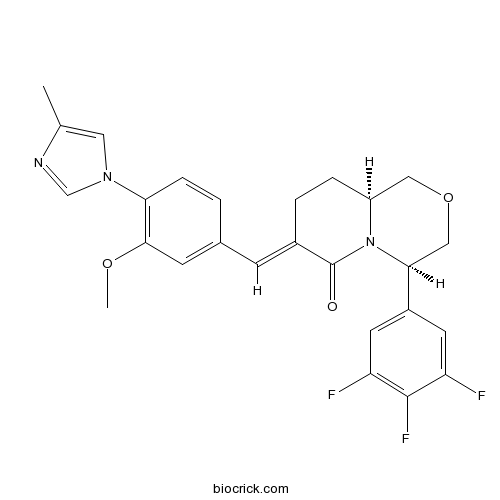
| Chemical Name | (4R,7E,9aS)-7-[[3-methoxy-4-(4-methylimidazol-1-yl)phenyl]methylidene]-4-(3,4,5-trifluorophenyl)-1,3,4,8,9,9a-hexahydropyrido[2,1-c][1,4]oxazin-6-one | ||
| SMILES | CC1=CN(C=N1)C2=C(C=C(C=C2)C=C3CCC4COCC(N4C3=O)C5=CC(=C(C(=C5)F)F)F)OC | ||
| Standard InChIKey | VHNYOQKVZQVBLC-RTCGXNAVSA-N | ||
| Standard InChI | InChI=1S/C26H24F3N3O3/c1-15-11-31(14-30-15)22-6-3-16(8-24(22)34-2)7-17-4-5-19-12-35-13-23(32(19)26(17)33)18-9-20(27)25(29)21(28)10-18/h3,6-11,14,19,23H,4-5,12-13H2,1-2H3/b17-7+/t19-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | gamma-Secretase Modulators (Amyloid-β production inhibitor) is a Amyloid-β production inhibitor. gamma-Secretase Modulators is useful for Alzheimer's disease.
IC50 value:
Target: γ-secretase modulator References: | |||||

gamma-Secretase Modulators Dilution Calculator

gamma-Secretase Modulators Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0683 mL | 10.3415 mL | 20.683 mL | 41.3659 mL | 51.7074 mL |
| 5 mM | 0.4137 mL | 2.0683 mL | 4.1366 mL | 8.2732 mL | 10.3415 mL |
| 10 mM | 0.2068 mL | 1.0341 mL | 2.0683 mL | 4.1366 mL | 5.1707 mL |
| 50 mM | 0.0414 mL | 0.2068 mL | 0.4137 mL | 0.8273 mL | 1.0341 mL |
| 100 mM | 0.0207 mL | 0.1034 mL | 0.2068 mL | 0.4137 mL | 0.5171 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxadiazine is a potent modulator of γ-secretase with Aβ42 IC50 value of 11 nM and Aβtotal/ Aβ42 value of 1170.[1]
γ-Secretase is one of intramenbrane-cleaving aspartyl protease which cleaves many type-I membrane proteins and many of them have important biological functions.γ-secretase is responsible for the generation Aβfrom the amyloid precusor protein.γ-Secretase has been considered as an important drug target for Alzheimer's disease.γ-Secertase also is responsible for Notch processing which is related to cancer such as leukemia.
Oxadiazine is one analogues of BMS-869780 which is anotherγ-secretase modulator. It showed a four folds separation between the IC 50 of Aβ42 and Aβ40 H4-APPsw cells which overexpress APP protein. It also increase Aβ37 and Aβ38 levels whereas decrease Aβ42 and Aβ40 level. It also has little effect on APP-CTFα and no effect on APP-CTFβ and notch processing. It can significantly decrease Aβ42 and Aβ40 level in rat brain and plasma.[1, 2]
References:
[1]. Huang X, Zhou W, Liu X, Li H, Sun G, Mandal M, Vicarel M, Zhu X, Bennett C, McCraken T et al: Synthesis and SAR Studies of Fused Oxadiazines as gamma-Secretase Modulators for Treatment of Alzheimer's Disease. ACS Med Chem Lett, 3(11):931-935.
[2]. Toyn JH, Thompson LA, Lentz KA, Meredith JE, Jr., Burton CR, Sankaranararyanan S, Guss V, Hall T, Iben LG, Krause CM et al: Identification and Preclinical Pharmacology of the gamma-Secretase Modulator BMS-869780. Int J Alzheimers Dis, 2014:431858.
- Neurodazine
Catalog No.:BCC7738
CAS No.:937807-66-4
- Neogambogic acid
Catalog No.:BCN2321
CAS No.:93772-31-7
- Jangomolide
Catalog No.:BCN4483
CAS No.:93767-25-0
- GRP (human)
Catalog No.:BCC5810
CAS No.:93755-85-2
- Magnaldehyde D
Catalog No.:BCN4070
CAS No.:93753-33-4
- Pacritinib (SB1518)
Catalog No.:BCC4558
CAS No.:937272-79-2
- SB1317
Catalog No.:BCC1925
CAS No.:937270-47-8
- ARRY-380
Catalog No.:BCC3726
CAS No.:937265-83-3
- NSC 95397
Catalog No.:BCC7109
CAS No.:93718-83-3
- GSK690693
Catalog No.:BCC2483
CAS No.:937174-76-0
- Leuconolam
Catalog No.:BCN4482
CAS No.:93710-27-1
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- Roxatidine Acetate HCl
Catalog No.:BCC4534
CAS No.:93793-83-0
- 3-Prenyl-2,4,6-trihydroxybenzophenone
Catalog No.:BCN1303
CAS No.:93796-20-4
- 22-beta-Acetoxyglycyrrhizin
Catalog No.:BCN7904
CAS No.:938042-17-2
- ATPγS tetralithium salt
Catalog No.:BCC7855
CAS No.:93839-89-5
- KU-0063794
Catalog No.:BCC2484
CAS No.:938440-64-3
- Isochamaejasmine
Catalog No.:BCN3128
CAS No.:93859-63-3
- 9-Oxo-2,7-bisaboladien-15-oic acid
Catalog No.:BCN4484
CAS No.:93888-59-6
- FIPI
Catalog No.:BCC7721
CAS No.:939055-18-2
- Hirsutanonol 5-O-glucoside
Catalog No.:BCN4485
CAS No.:93915-36-7
- Toonaciliatin M
Catalog No.:BCN7881
CAS No.:93930-04-2
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
Discovery of novel 5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridine derivatives as gamma-secretase modulators (Part 2).[Pubmed:27255179]
Bioorg Med Chem. 2016 Jul 15;24(14):3192-206.
gamma-Secretase Modulators (GSMs), which lower pathogenic amyloid beta (Abeta) without affecting the production of total Abeta or Notch signal, have emerged as a potential therapeutic agent for Alzheimer's disease (AD). A novel series of 5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridine derivatives was discovered and characterized as GSMs. Optimization of substituents at the 8-position of the core scaffold using ligand-lipophilicity efficiency (LLE) as a drug-likeness guideline led to identification of various types of high-LLE GSMs. Phenoxy compound (R)-17 exhibited especially high LLE as well as potent in vivo Abeta42-lowering effect by single administration. Furthermore, multiple oral administration of (R)-17 significantly reduced soluble and insoluble brain Abeta42, and ameliorated cognitive deficit in novel object recognition test (NORT) using Tg2576 mice as an AD model.
Design and synthesis of aminothiazole modulators of the gamma-secretase enzyme.[Pubmed:27426299]
Bioorg Med Chem Lett. 2016 Aug 15;26(16):3928-37.
The design and construction of a series of novel aminothiazole-derived gamma-Secretase Modulators is described. The incorporation of heterocyclic replacements of the terminal phenyl D-ring of lead compound 1 was conducted in order to align potency with favorable drug-like properties. gamma-Secretase modulator 28 displayed good activity for in vitro inhibition of Abeta42, as well as substantial improvement in ADME and physicochemical properties, including aqueous solubility. Pharmacokinetic evaluation of compound 28 in mice revealed good brain penetration, as well as good clearance, half-life, and volume of distribution which collectively support the continued development of this class of compounds.
Analyzing Amyloid-beta Peptide Modulation Profiles and Binding Sites of gamma-Secretase Modulators.[Pubmed:28065262]
Methods Enzymol. 2017;584:157-183.
gamma-Secretase is a key player in the pathogenesis of Alzheimer's disease (AD). The intramembrane-cleaving enzyme initially cleaves a C-terminal fragment of the amyloid precursor protein (APP) at the varepsilon-site within its transmembrane domain to release the APP intracellular domain. Subsequent stepwise carboxy-terminal trimming cleavages eventually release amyloid-beta (Abeta) peptides of 37-43 amino acids into the extracellular space. Abeta42 as well as the much less abundant Abeta43 species are highly aggregation prone and can deposit as plaques in the brains of affected patients, which are widely believed to be causative of AD. Disappointingly, due to lack of efficacy and side effects likely attributable to the inhibition of the crucial substrate Notch, inhibitors of gamma-secretase that lower Abeta generation failed in clinical trials of AD. There is hope, however, that recently developed potent gamma-Secretase Modulators (GSMs) provide a safer approach for disease modification. These compounds have the unique property of primarily shifting the generation of Abeta42 toward that of shorter peptides without affecting the varepsilon-site cleavage of Notch and other substrates. In this chapter, we describe methods to investigate how GSMs affect the activity of the enzyme as well as how their molecular targets are identified.
Design, Synthesis, and Evaluation of a Novel Series of Oxadiazine Gamma Secretase Modulators for Familial Alzheimer's Disease.[Pubmed:28230986]
J Med Chem. 2017 Mar 23;60(6):2383-2400.
Herein we describe the design, synthesis, and evaluation of a novel series of oxadiazine-based gamma secretase modulators obtained via isosteric amide replacement and critical consideration of conformational restriction. Oxadiazine lead 47 possesses good in vitro potency with excellent predicted CNS drug-like properties and desirable ADME/PK profile. This lead compound demonstrated robust Abeta42 reductions and subsequent Abeta37 increases in both rodent brain and CSF at 30 mg/kg dosed orally.


