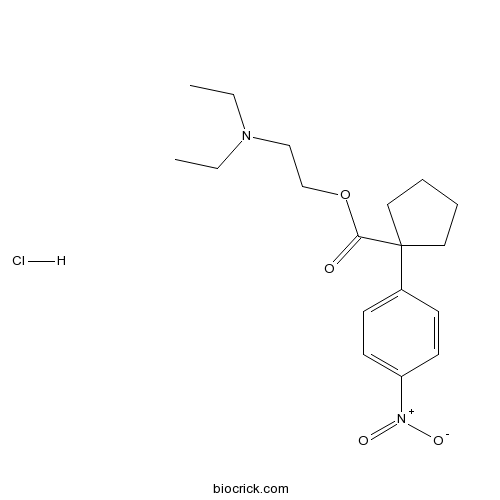
Nitrocaramiphen hydrochlorideMuscarinic antagonist, M1 > M2 CAS# 98636-73-8 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98636-73-8 | SDF | Download SDF |
| PubChem ID | 25102587 | Appearance | Powder |
| Formula | C18H27ClN2O4 | M.Wt | 370.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | 2-(diethylamino)ethyl 1-(4-nitrophenyl)cyclopentane-1-carboxylate;hydrochloride | ||
| SMILES | CCN(CC)CCOC(=O)C1(CCCC1)C2=CC=C(C=C2)[N+](=O)[O-].Cl | ||
| Standard InChIKey | XWQWACGTGFICFO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H26N2O4.ClH/c1-3-19(4-2)13-14-24-17(21)18(11-5-6-12-18)15-7-9-16(10-8-15)20(22)23;/h7-10H,3-6,11-14H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Muscarinic antagonist with 71-fold selectivity for M1 over M2. |

Nitrocaramiphen hydrochloride Dilution Calculator

Nitrocaramiphen hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6963 mL | 13.4814 mL | 26.9629 mL | 53.9258 mL | 67.4072 mL |
| 5 mM | 0.5393 mL | 2.6963 mL | 5.3926 mL | 10.7852 mL | 13.4814 mL |
| 10 mM | 0.2696 mL | 1.3481 mL | 2.6963 mL | 5.3926 mL | 6.7407 mL |
| 50 mM | 0.0539 mL | 0.2696 mL | 0.5393 mL | 1.0785 mL | 1.3481 mL |
| 100 mM | 0.027 mL | 0.1348 mL | 0.2696 mL | 0.5393 mL | 0.6741 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sarmentocymarin
Catalog No.:BCN7489
CAS No.:98633-61-5
- (S)-(-)-Bay K 8644
Catalog No.:BCC7108
CAS No.:98625-26-4
- Schisandrone
Catalog No.:BCN3316
CAS No.:98619-25-1
- Cryptanoside A
Catalog No.:BCN7481
CAS No.:98570-81-1
- Sominone
Catalog No.:BCN8006
CAS No.:98569-64-3
- 4-Chloro-6-iodoquinazoline
Catalog No.:BCC8703
CAS No.:98556-31-1
- Methyl 2-bromomethyl-3-nitrobenzoate
Catalog No.:BCC9035
CAS No.:98475-07-1
- Pseudoginsenoside RT5
Catalog No.:BCN1076
CAS No.:98474-78-3
- Pseudoginsenoside RT1
Catalog No.:BCN2794
CAS No.:98474-74-9
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
- Metoprolol Succinate
Catalog No.:BCC6519
CAS No.:98418-47-4
- Finasteride
Catalog No.:BCC2491
CAS No.:98319-26-7
- Zedoarondiol
Catalog No.:BCN3560
CAS No.:98644-24-7
- Methyl ganoderate H
Catalog No.:BCN3258
CAS No.:98665-11-3
- Ganoderic acid F
Catalog No.:BCN3037
CAS No.:98665-14-6
- Ganoderic Acid J
Catalog No.:BCN8436
CAS No.:100440-26-4
- Lucidenic acid D2
Catalog No.:BCN8202
CAS No.:98665-16-8
- Ganoderic acid H
Catalog No.:BCN3038
CAS No.:98665-19-1
- Ganoderic acid I
Catalog No.:BCN2865
CAS No.:98665-20-4
- Ganolucidic acid A
Catalog No.:BCN2444
CAS No.:98665-21-5
- Ganoderic acid G
Catalog No.:BCN2915
CAS No.:98665-22-6
- Dregeoside Da1
Catalog No.:BCN4764
CAS No.:98665-65-7
- Dregeoside Ga1
Catalog No.:BCN4548
CAS No.:98665-66-8
- ATP disodium salt
Catalog No.:BCC5160
CAS No.:987-65-5
Caramiphen, iodocaramiphen and nitrocaramiphen are potent, competitive, muscarinic M1 receptor-selective agents.[Pubmed:8449241]
Eur J Pharmacol. 1993 Feb 16;231(3):485-8.
Caramiphen, iodocaramiphen and nitrocaramiphen were examined for affinity at the muscarinic M1, M2 and M3 receptor subtypes in radioligand binding assays. Caramiphen binds with high affinity at the M1 site labeled by [3H]pirenzepine in rat cortex (Ki = 1.2 nM) and displays a 27-fold greater preference for the M1 than the M2 site labeled by [3H](-)-quinuclidinyl benzilate in rat heart, and a 6-fold greater preference for the M1 than the M3 site labeled by [3H]N-methylscopolamine in rat submaxillary gland. Iodocaramiphen binds with high affinity (Ki = 2.1 nM) and selectivity (59-fold) for the M1 vs. M2 subtype, and is 4-fold more selective for the M1 vs. M3 site. Nitrocaramiphen binds with high affinity for M1 sites (Ki = 5.5 nM) and with a 71-fold selectivity over M2, and a 10-fold selectivity for the M1 over the M3 subtype. All three compounds interacted with the M1 binding site in a competitive manner. Nitrocaramiphen and iodocaramiphen are as potent and showed a comparable selectivity for binding to the M1 over the M2 site than the prototypical agent pirenzepine (M1; Ki = 5.2 nM, 51-fold selectivity). Additionally, nitrocaramiphen demonstrates at least a 10-fold selectivity for the M1 over the M3 site. These ester-type antimuscarinics may be better ligands for the study of M1 receptors in brain than the hydrophilic agent pirenzepine.
Muscarinic receptor binding profile of para-substituted caramiphen analogues.[Pubmed:1920350]
J Med Chem. 1991 Oct;34(10):2984-9.
Para-substituted analogues of the antimuscarinic agent caramiphen were synthesized and evaluated for their ability to bind to the M1 and M2 subtypes of the muscarinic receptor. The purpose of the set was to look for a possible relationship in binding affinity or receptor subtype selectivity with aromatic substituent parameters such as Hammett's sigma or Hansch's pi values. It is felt this could be determined initially with only four properly chosen substituents. In this approach, substituents were chosen which have an extreme value for sigma and for pi, in a positive and negative direction, in all combinations. The substituents chosen for examination were amino (-sigma, -pi); 1-pyrrolidinyl (-sigma, +pi); 1-tetrazolyl (+sigma, -pi), and iodo (+sigma, +pi). It was determined in this research that caramiphen binds with high affinity (Ki = 1.2 nM) and is selective for the M1 over M2 muscarinic receptor subtype (26-fold). An examination of para-substitution reveals that compounds with electron-withdrawing (+sigma) substituents showed M1 selectivity, while the derivatives with electron-donating groups (-sigma) were nonselective in the binding assays. On the basis of this finding, the nitro and cyano derivatives were prepared and found to be M1 selective. The + sigma derivatives showed a decrease in M2 affinity while the p-nitro and p-iodo derivatives retained approximately equal affinity as caramiphen for the M1 site. The nitro- and iodocaramiphen derivatives were as potent (M1, Ki = 5.52 and 2.11 nM, respectively) and showed a greater selectivity of M1 over M2 binding than the M1 prototypical agent pirenzepine (M1, Ki = 5.21 nM).


