OxfendazoleCAS# 53716-50-0 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53716-50-0 | SDF | Download SDF |
| PubChem ID | 40854 | Appearance | Powder |
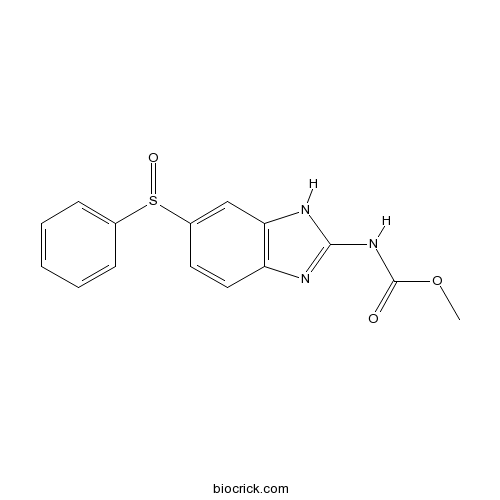
| Formula | C15H13N3O3S | M.Wt | 315.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (158.55 mM; Need ultrasonic) H2O : 0.67 mg/mL (2.12 mM; Need ultrasonic) | ||
| Chemical Name | methyl N-[6-(benzenesulfinyl)-1H-benzimidazol-2-yl]carbamate | ||
| SMILES | COC(=O)NC1=NC2=C(N1)C=C(C=C2)S(=O)C3=CC=CC=C3 | ||
| Standard InChIKey | BEZZFPOZAYTVHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13N3O3S/c1-21-15(19)18-14-16-12-8-7-11(9-13(12)17-14)22(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,16,17,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxfendazole Dilution Calculator

Oxfendazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1711 mL | 15.8554 mL | 31.7108 mL | 63.4216 mL | 79.277 mL |
| 5 mM | 0.6342 mL | 3.1711 mL | 6.3422 mL | 12.6843 mL | 15.8554 mL |
| 10 mM | 0.3171 mL | 1.5855 mL | 3.1711 mL | 6.3422 mL | 7.9277 mL |
| 50 mM | 0.0634 mL | 0.3171 mL | 0.6342 mL | 1.2684 mL | 1.5855 mL |
| 100 mM | 0.0317 mL | 0.1586 mL | 0.3171 mL | 0.6342 mL | 0.7928 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxfendazole
- Carprofen
Catalog No.:BCC4645
CAS No.:53716-49-7
- Tubacin
Catalog No.:BCC2428
CAS No.:537049-40-4
- BML-210(CAY10433)
Catalog No.:BCC6479
CAS No.:537034-17-6
- 2-Acetyl-5-bromothiophene
Catalog No.:BCC8513
CAS No.:5370-25-2
- Mexiletine HCl
Catalog No.:BCC4677
CAS No.:5370-01-4
- beta-Amyrenonol methylthiomethyl ether
Catalog No.:BCN3354
CAS No.:
- N-Acetyl-m-toluidine
Catalog No.:BCC9083
CAS No.:537-92-8
- Soyasaponin Be Methyl Ester
Catalog No.:BCN5925
CAS No.:117210-13-6
- N-Acetyl-L-tyrosine
Catalog No.:BCC9082
CAS No.:537-55-3
- Pterostilbene
Catalog No.:BCN2539
CAS No.:537-42-8
- Chlorophorin
Catalog No.:BCN3288
CAS No.:537-41-7
- Convolvine
Catalog No.:BCN1904
CAS No.:537-30-4
- Bruceantinol
Catalog No.:BCN7616
CAS No.:53729-52-5
- Luteolin-7-O-glucoside
Catalog No.:BCN5388
CAS No.:5373-11-5
- Neorauflavane
Catalog No.:BCN4791
CAS No.:53734-74-0
- Neorauflavene
Catalog No.:BCN4848
CAS No.:53734-75-1
- Lyclaninol
Catalog No.:BCN5710
CAS No.:53755-76-3
- Lycernuic acid A
Catalog No.:BCN5711
CAS No.:53755-77-4
- UF 010
Catalog No.:BCC6478
CAS No.:537672-41-6
- CP-724714
Catalog No.:BCC1188
CAS No.:537705-08-1
- Ribostamycin Sulfate
Catalog No.:BCC4710
CAS No.:53797-35-6
- DCC
Catalog No.:BCC2810
CAS No.:538-75-0
- 1-Benzothiophene-3-carbaldehyde
Catalog No.:BCC8454
CAS No.:5381-20-4
- Onitin
Catalog No.:BCN5712
CAS No.:53823-02-2
Efficacy of ivermectin and oxfendazole against Taenia solium cysticercosis and other parasitoses in naturally infected pigs.[Pubmed:23806569]
Acta Trop. 2013 Oct;128(1):48-53.
Smallholder semi-confined pig production is a fast growing practice in sub-Saharan Africa with an unfortunate outcome of high prevalence of Taenia solium cysticercosis and other parasitoses. The widely used anthelmintic for control of endo and ecto-parasites in pigs in the area is ivermectin at a recommended dose of 0.3mg/kg. This study was conducted to evaluate the efficacy and safety in pigs after subcutaneous injection of ivermectin (IVM, 0.3mg/kg) and orally administration of Oxfendazole (OFZ, 30mg/kg) in treatment of porcine cysticercosis and other parasitoses in naturally infected pigs. A total of 61 pigs with T. solium cysticercosis (38 males and 23 females) as identified by tongue palpation with age ranging from 3 to 24 months were recruited. The pigs were stratified based on sex, age and number of cysts on the tongue and randomly allocated to IVM, OFZ and control groups. Three days before treatment and two weeks after treatment faecal samples and skin scrapings were taken to establish the burden of endo- and ectoparasites, respectively and the effect of the treatment. No adverse effect was observed in any of the treatment groups throughout the study period. Half of the pigs from each group were slaughtered at week four and the remaining half at week twelve post treatment. The IVM treatment group had no significant effect (p=0.224) on T. solium cysts viability in comparison to the control group. Significant effect on cysts viability was observed in the OFZ treated group (p<0.001) compared to IVM and control groups in all muscle tissues. Regarding to brain cysts, neither of the drugs was efficacious. Ivermectin and OFZ treatments significantly reduced (p<0.001) the faecal egg count of Ascaris suum, strongyles and Trichuris suis two weeks after treatment. At slaughter, Oesophagostomum dentatum, Ascarops strongylina and Physocephalus sexalatus were recovered from pigs in the IVM treated and in the control groups. Ivermectin was 100% effective in control of Sarcoptes scabiei. In conclusion, IVM at a single dose of 0.3mg/kg was efficacious against ectoparasites but did not effectively cure pigs from T. solium cysticercosis or nematodes. Oxfendazole, on the other hand, killed all nematodes and muscle cysts, but did not have any effect on ectoparasites. A combination of the two drugs would be a most useful treatment option for control of pig parasitoses in sub-Saharan Africa.
Oxfendazole flukicidal activity in pigs.[Pubmed:24713198]
Acta Trop. 2014 Aug;136:10-3.
Although Oxfendazole (OFZ) is a well know broad-spectrum benzimidazole anthelmintic, the assessment of its potential trematodicidal activity remains unexplored. OFZ administration at single high doses has been recommended to control Taenia solium cysticercus in pigs. The current study investigated the flukicidal activity obtained after a single high (30mg/kg) oral dose of OFZ in pigs harbouring a natural Fasciola hepatica infection. Sixteen (16) local ecotype pigs were randomly allocated into two (2) experimental groups of 8 animals each named as follow: Untreated control and OFZ treated, in which animals received OFZ (Synanthic((R)), Merial Ltd., 9.06% suspension) orally at 30mg/kg. At seven (7) days post-treatment, all the animals were sacrificed and direct adult liver fluke counts were performed following the WAAVP guidelines. None of the animals involved in this experiment showed any adverse event during the study. OFZ treatment as a single 30mg/kg oral dose showed a 100% efficacy against F. hepatica. In conclusion, the trial described here demonstrated an excellent OFZ activity against F. hepatica in naturally infected pigs, after its administration at a single oral dose of 30mg/kg.
Preclinical studies on the pharmacokinetics, safety, and toxicology of oxfendazole: toward first in human studies.[Pubmed:25701764]
Int J Toxicol. 2015 Mar-Apr;34(2):129-37.
A 2-week study in rats identified target organs of Oxfendazole toxicity to be bone marrow, epididymis, liver, spleen, testis, and thymus. Female rats had greater Oxfendazole exposure and exhibited toxicities at lower doses than did males. Decreased white blood cell levels, a class effect of benzimidazole anthelmintics, returned to normal during the recovery period. The no observed adverse effect level was determined to be >5 but <25 mg/kg/d and the maximum tolerated dose 100 mg/kg/d. The highest dose, 200 mg/kg/d, resulted in significant toxicity and mortality, leading to euthanization of the main study animals in this group after 7 days. Oxfendazole did not exhibit genetic toxicology signals in standard Ames bacterial, mouse lymphoma, or rat micronucleus assays nor did it provoke safety concerns when evaluated for behavioral effects in rats or cardiovascular safety effects in dogs. These results support the transition of Oxfendazole to First in Human safety studies preliminary to its evaluation in human helminth diseases.
Simultaneous determination of praziquantel, pyrantel embonate, febantel and its active metabolites, oxfendazole and fenbendazole, in dog plasma by liquid chromatography/mass spectrometry.[Pubmed:26104502]
Biomed Chromatogr. 2015 Dec;29(12):1859-65.
A liquid chromatography-electrospray-mass spectrometry method (LC/MS) has been developed and validated for determination of praziquantel (PZQ), pyrantel (PYR), febantel (FBT), and the active metabolites fenbendazole (FEN) and Oxfendazole (OXF), in dog plasma, using mebendazole as internal standard (IS). The method consists of solid-phase extractions on Strata-X polymeric cartridges. Chromatographic separation was carried out on a Phenomenex Gemini C6 -Phenyl column using binary gradient elution containing methanol and 50 mm ammonium-formate (pH 3). The method was linear (r(2) >/= 0.990) over concentration ranges of 3-250 ng/mL for PYR andFEB, 5-250 ng/mL for OXF and FEN, and 24-1000 ng/mL for PZQ. The mean precisions were 1.3-10.6% (within-run) and 2.5-9.1% (between-run), and mean accuracies were 90.7-109.4% (within-run) and 91.6-108.2% (between-run). The relative standard deviations (RSD) were <9.1%. The mean recoveries of five targeted compounds from dog plasma ranged from 77 to 94%.The new LC/MS method described herein was fully validated and successfully applied to the bioequivalence studies of different anthelmintic formulations such as tablets containing PZQ, PYR embonate and FBT in dogs after oral administration.


