PAP-1Kv1.3 inhibitor CAS# 870653-45-5 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

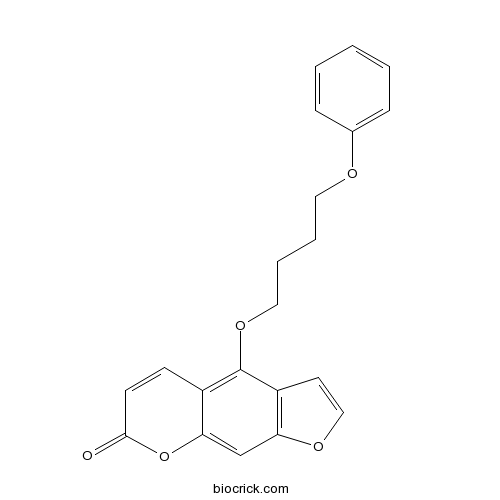
| Cas No. | 870653-45-5 | SDF | Download SDF |
| PubChem ID | 11302540 | Appearance | Powder |
| Formula | C21H18O5 | M.Wt | 350.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-(4-Phenoxybutoxy)psoralen | ||
| Solubility | DMSO : 50 mg/mL (142.71 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 4-(4-phenoxybutoxy)furo[3,2-g]chromen-7-one | ||
| SMILES | C1=CC=C(C=C1)OCCCCOC2=C3C=CC(=O)OC3=CC4=C2C=CO4 | ||
| Standard InChIKey | KINMYBBFQRSVLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H18O5/c22-20-9-8-16-19(26-20)14-18-17(10-13-24-18)21(16)25-12-5-4-11-23-15-6-2-1-3-7-15/h1-3,6-10,13-14H,4-5,11-12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PAP-1 is a selective inhibitor of Kv1.3, voltage-gated K+ channel. PAP-1 (EC50=2 nM) potently inhibits human T effector memory cell proliferation and delayed hypersensitivity.
IC50 value: 2 nM (EC50) [1]
in vitro: blocks Kv1.3 in a use-dependent manner, with a Hill coefficient of 2 and an EC50 of 2 nM, by preferentially binding to the C-type inactivated state of the channel. PAP-1 is 23-fold selective over Kv1.5, 33- to 125-fold selective over other Kv1-family channels, and 500- to 7500-fold selective over Kv2.1, Kv3.1, Kv3.2, Kv4.2, HERG, calcium-activated K+ channels, Na+,Ca2+, and Cl- channels [1]. The blockade of Kv1.3 results in membrane depolarization and inhibition of TEM proliferation and function. In this study, the in vitro effects of PAP-1 on T cells and the in vivo toxicity and pharmacokinetics (PK) were examined in rhesus macaques (RM) with the ultimate aim of utilizing PAP-1 to define the role of TEMs in RM infected with simian immunodeficiency virus (SIV). Electrophysiologic studies on T cells in RM revealed a Kv1.3 expression pattern similar to that in human T cells. Thus, PAP-1 effectively suppressed TEM proliferation in RM [2].
in vivo: PAP-1 does not exhibit cytotoxic or phototoxic effects, is negative in the Ames test, and affects cytochrome P450-dependent enzymes only at micromolar concentrations. PAP-1 potently inhibits the proliferation of human TEM cells and suppresses delayed type hypersensitivity, a TEM cell-mediated reaction, in rats [1]. When administered intravenously, PAP-1 showed a half-life of 6.4 hrs; the volume of distribution suggested extensive distribution into extravascular compartments. When orally administered, PAP-1 was efficiently absorbed. Plasma concentrations in RM undergoing a 30-day, chronic dosing study indicated that PAP-1 levels suppressive to TEMs in vitro can be achieved and maintained in vivo at a non-toxic dose [2]. References: | |||||

PAP-1 Dilution Calculator

PAP-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8541 mL | 14.2706 mL | 28.5413 mL | 57.0825 mL | 71.3531 mL |
| 5 mM | 0.5708 mL | 2.8541 mL | 5.7083 mL | 11.4165 mL | 14.2706 mL |
| 10 mM | 0.2854 mL | 1.4271 mL | 2.8541 mL | 5.7083 mL | 7.1353 mL |
| 50 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1417 mL | 1.4271 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2854 mL | 0.5708 mL | 0.7135 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PAP-1 is selective inhibitor of Kv1.3, voltage-gated K+ channel. PAP-1 (EC50=2 nM) potently inhibits human T effector memory cell proliferation and delayed hypersensitivity. Effective orally or intraperitoneally. 5-(4-Phenoxybutoxy)psoralen has 23-fold selectivity for Kv1.3 over Kv1.5, and 33-125-fold selectivity over other Kv1 family channels. In treatment of multiple sclerosis
- Euphohelioscopin A
Catalog No.:BCN6501
CAS No.:87064-61-7
- Zeylasteral
Catalog No.:BCN3065
CAS No.:87064-16-2
- Ritanserin
Catalog No.:BCC7214
CAS No.:87051-43-2
- GW2580
Catalog No.:BCC1096
CAS No.:870483-87-7
- Bis-5,5-nortrachelogenin
Catalog No.:BCN6516
CAS No.:870480-56-1
- 3,23-Dioxo-9,19-cyclolanost-24-en-26-oic acid
Catalog No.:BCN1322
CAS No.:870456-88-5
- CAL-101 (Idelalisib, GS-1101)
Catalog No.:BCC1270
CAS No.:870281-82-6
- Acalisib (GS-9820)
Catalog No.:BCC6384
CAS No.:870281-34-8
- Apilimod mesylate
Catalog No.:BCC5287
CAS No.:870087-36-8
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
- A 839977
Catalog No.:BCC4290
CAS No.:870061-27-1
- xylitol pentacetate
Catalog No.:BCN6267
CAS No.:13437-68-8
- AC 261066
Catalog No.:BCC7848
CAS No.:870773-76-5
- Leptomycin B
Catalog No.:BCC7223
CAS No.:87081-35-4
- MK 0893
Catalog No.:BCC1752
CAS No.:870823-12-4
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
- Mulberrofuran G
Catalog No.:BCN3693
CAS No.:87085-00-5
- 5-Hydroxy-1,7-diphenyl-6-hepten-3-one
Catalog No.:BCN1321
CAS No.:87095-74-7
- Neuromedin B (porcine)
Catalog No.:BCC5841
CAS No.:87096-84-2
- 5-Methoxystrictamine
Catalog No.:BCN4416
CAS No.:870995-64-5
- TAK-285
Catalog No.:BCC3860
CAS No.:871026-44-7
- Raltegravir potassium salt
Catalog No.:BCC5457
CAS No.:871038-72-1
- TCS 3035
Catalog No.:BCC8036
CAS No.:871085-49-3
- Almorexant
Catalog No.:BCC5122
CAS No.:871224-64-5
Stimulation of glucose uptake in murine soleus muscle and adipocytes by 5-(4-phenoxybutoxy)psoralen (PAP-1) may be mediated by Kv1.5 rather than Kv1.3.[Pubmed:25320682]
PeerJ. 2014 Oct 7;2:e614.
Kv1 channels are shaker-related potassium channels that influence insulin sensitivity. Kv1.3(-/-) mice are protected from diet-induced insulin resistance and some studies suggest that Kv1.3 inhibitors provide similar protection. However, it is unclear whether blockade of Kv1.3 in adipocytes or skeletal muscle increases glucose uptake. There is no evidence that the related channel Kv1.5 has any influence on insulin sensitivity and its expression in adipose tissue has not been reported. PAP-1 is a selective inhibitor of Kv1.3, with 23-fold, 32-fold and 125-fold lower potencies as an inhibitor of Kv1.5, Kv1.1 and Kv1.2 respectively. Soleus muscles from wild-type and genetically obese ob/ob mice were incubated with 2-deoxy[1-(14)C]-glucose for 45 min and formation of 2-deoxy[1-(14)C]-glucose-6-phosphate was measured. White adipocytes were incubated with D-[U-(14)C]-glucose for 1 h. TNFalpha and Il-6 secretion from white adipose tissue pieces were measured by enzyme-linked-immunoassay. In the absence of insulin, a high concentration (3 microM) of PAP-1 stimulated 2-deoxy[1-14C]-glucose uptake in soleus muscle of wild-type and obese mice by 30% and 40% respectively, and in adipocytes by 20% and 50% respectively. PAP-1 also stimulated glucose uptake by adipocytes at the lower concentration of 1 microM, but at 300 nM, which is still 150-fold higher than its EC50 value for inhibition of the Kv1.3 channel, it had no effect. In the presence of insulin, PAP-1 (3 microM) had a significant effect only in adipocytes from obese mice. PAP-1 (3 microM) reduced the secretion of TNFalpha by adipose tissue but had no effect on the secretion of IL-6. Expression of Kv1.1, Kv1.2, Kv1.3 and Kv1.5 was determined by RT-PCR. Kv1.3 and Kv1.5 mRNA were detected in liver, gastrocnemius muscle, soleus muscle and white adipose tissue from wild-type and ob/ob mice, except that Kv1.3 could not be detected in gastrocnemius muscle, nor Kv1.5 in liver, of wild-type mice. Expression of both genes was generally higher in liver and muscle of ob/ob mice compared to wild-type mice. Kv1.5 appeared to be expressed more highly than Kv1.3 in soleus muscle, adipose tissue and adipocytes of wild-type mice. Expression of Kv1.2 appeared to be similar to that of Kv1.3 in soleus muscle and adipose tissue, but Kv1.2 was undetectable in adipocytes. Kv1.1 could not be detected in soleus muscle, adipose tissue or adipocytes. We conclude that inhibition of Kv1 channels by PAP-1 stimulates glucose uptake by adipocytes and soleus muscle of wild-type and ob/ob mice, and reduces the secretion of TNFalpha by adipose tissue. However, these effects are more likely due to inhibition of Kv1.5 than to inhibition of Kv1.3 channels.
K+ channels expression in hypertension after arterial injury, and effect of selective Kv1.3 blockade with PAP-1 on intimal hyperplasia formation.[Pubmed:25348824]
Cardiovasc Drugs Ther. 2014 Dec;28(6):501-11.
INTRODUCTION: K(+) channels are central to vascular pathophysiology. Previous results demonstrated that phenotypic modulation associates with a change in Kv1.3 to Kv1.5 expression, and that Kv1.3 blockade inhibits proliferation of VSMCs cultures. PURPOSE: To explore whether the Kv1.3 to Kv1.5 switch could be a marker of the increased risk of intimal hyperplasia in essential hypertension and whether systemic treatment with Kv1.3 blockers can prevent intimal hyperplasia after endoluminal lesion . METHODS: Morphometric and immunohistochemical analysis were performed in arterial segments following arterial injury and constant infusion of the Kv1.3 blocker PAP-1 during 28 days. Differential expression of K(+) channel genes was studied in VSMC from hypertensive (BPH) and normotensive (BPN) mice, both in control and after endoluminal lesion. Finally, the migration and proliferation rate of BPN and BPH VSMCs was explored in vitro. RESULTS: Changes in mRNA expression led to an increased Kv1.3/Kv1.5 ratio in BPH VSMC. Consistent with this, arterial injury in BPH mice induced a higher degree of luminal stenosis, (84 +/- 4% vs. 70 +/- 5% in BPN, p < 0.01), although no differences in migration and proliferation rate were observed in cultured VSMCs. The in vivo proliferative lesions were significantly decreased upon PAP-1 systemic infusion (18 +/- 6% vs. 58 +/- 20% with vehicle, p < 0.05). CONCLUSIONS: Hypertension leads to a higher degree of luminal stenosis in our arterial injury model, that correlates with a decreased expression of Kv1.5 channels. Kv1.3 blockers decreased in vitro VSMCs proliferation, migration, and in vivo intimal hyperplasia formation, pointing to Kv1.3 channels as promising therapeutical targets against restenosis.
In silico identification of PAP-1 binding sites in the Kv1.2 potassium channel.[Pubmed:25734225]
Mol Pharm. 2015 Apr 6;12(4):1299-307.
Voltage-gated potassium channels of the Kv1 family play a crucial role in the generation and transmission of electrical signals in excitable cells affecting neuronal and cardiac activities. Small-molecule blockage of these channels has been proposed to occur via a cooperative mechanism involving two main blocking sites: the inner-pore site located below the selectivity filter, and a side-pocket cavity located between the pore and the voltage sensor. Using 0.5 mus molecular dynamics simulation trajectories complemented by docking calculations, the potential binding sites of the PAP-1 (5-(4-phenoxybutoxy)psoralen) blocker to the crystal structure of Kv1.2 channel have been studied. The presence of both mentioned blocking sites at Kv1.2 is confirmed, adding evidence in favor of a cooperative channel blockage mechanism. These observations provide insight into drug modulation that will guide further developments of Kv inhibitors.


