RS 45041-190 hydrochlorideCAS# 204274-74-8 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 204274-74-8 | SDF | Download SDF |
| PubChem ID | 10332716 | Appearance | Powder |
| Formula | C11H13Cl2N3 | M.Wt | 258.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
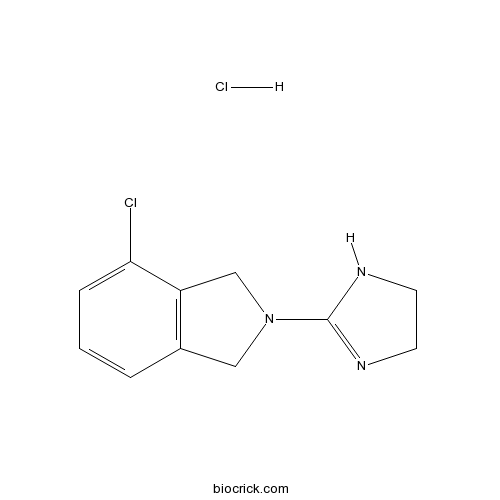
| Chemical Name | 4-chloro-2-(4,5-dihydro-1H-imidazol-2-yl)-1,3-dihydroisoindole;hydrochloride | ||
| SMILES | C1CN=C(N1)N2CC3=C(C2)C(=CC=C3)Cl.Cl | ||
| Standard InChIKey | WRYJNVYEPVATQV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H12ClN3.ClH/c12-10-3-1-2-8-6-15(7-9(8)10)11-13-4-5-14-11;/h1-3H,4-7H2,(H,13,14);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective I2 imidazoline receptor ligand. |

RS 45041-190 hydrochloride Dilution Calculator

RS 45041-190 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8737 mL | 19.3686 mL | 38.7372 mL | 77.4743 mL | 96.8429 mL |
| 5 mM | 0.7747 mL | 3.8737 mL | 7.7474 mL | 15.4949 mL | 19.3686 mL |
| 10 mM | 0.3874 mL | 1.9369 mL | 3.8737 mL | 7.7474 mL | 9.6843 mL |
| 50 mM | 0.0775 mL | 0.3874 mL | 0.7747 mL | 1.5495 mL | 1.9369 mL |
| 100 mM | 0.0387 mL | 0.1937 mL | 0.3874 mL | 0.7747 mL | 0.9684 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
- Boc-Ser-OH.H2O
Catalog No.:BCC2599
CAS No.:204191-40-2
- Caesalmin E
Catalog No.:BCN7247
CAS No.:204185-91-1
- 4'-Hydroxy-2-O-methylanigorufone
Catalog No.:BCN7179
CAS No.:204134-70-3
- Anabasamine
Catalog No.:BCN2148
CAS No.:20410-87-1
- (2-Aminoethyl)phosphonic acid
Catalog No.:BCN1762
CAS No.:2041-14-7
- PD 176252
Catalog No.:BCC7426
CAS No.:204067-01-6
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
- Helioxanthin derivative 5-4-2
Catalog No.:BCC5414
CAS No.:203935-39-1
- Fmoc-ß-HoGlu(OtBu)-OH
Catalog No.:BCC3234
CAS No.:203854-49-3
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- 27-Hydroxycholesterol
Catalog No.:BCN2750
CAS No.:20380-11-4
- NS 2028
Catalog No.:BCC6212
CAS No.:204326-43-2
- Fmoc-β-Homo-Phe-OH
Catalog No.:BCC2629
CAS No.:204384-69-0
- BCH
Catalog No.:BCC7993
CAS No.:20448-79-7
- Talnetant hydrochloride
Catalog No.:BCC1982
CAS No.:204519-66-4
- Epitaraxerol
Catalog No.:BCN4677
CAS No.:20460-33-7
- Olcegepant
Catalog No.:BCC1818
CAS No.:204697-65-4
- Fmoc-Lys(ivDde)-OH
Catalog No.:BCC3520
CAS No.:204777-78-6
- 8-O-Acetyltorilolone
Catalog No.:BCN7094
CAS No.:20482-21-7
- Procumbide
Catalog No.:BCN3932
CAS No.:20486-27-5
- Nardosinonediol
Catalog No.:BCN8118
CAS No.:20489-11-6
- Curzerenone
Catalog No.:BCN3009
CAS No.:20493-56-5
- 3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN1503
CAS No.:204935-85-3
RS-45041-190: a selective, high-affinity ligand for I2 imidazoline receptors.[Pubmed:8528553]
Br J Pharmacol. 1995 Sep;116(2):1737-44.
1. RS-45041-190 (4-chloro-2-(imidazolin-2-yl)isoindoline) showed high affinity for I2 imidazoline receptors labelled by [3H]-idazoxan in rat (pKi = 8.66 +/- 0.09), rabbit (pKi = 9.37 +/- 0.07), dog (pKi = 9.32 +/- 0.18) and baboon kidney (pKi = 8.85 +/- 0.12), but had very low affinity for alpha 2-adrenoceptors in rat cerebral cortex (pKi = 5.7 +/- 0.09). 2. RS-45041-190 showed low affinity for other adrenoceptors, dopamine, 5-hydroxytryptamine, and muscarinic receptors and dihydropyridine binding sites (selectivity ratio > 1000). 3. RS-45041-190 showed moderate potency for the inhibition of monoamine oxidase A in vitro (pIC50 = 6.12), but had much lower potency for monoamine oxidase B (pIC50 = 4.47), neither of which equated with its affinity for I2 receptors. 4. RS-45041-190 (0.001 to 3 mg kg-1, i.v. and 1 ng-50 micrograms i.c.v.) had only small, transient effects on blood pressure and heart rate in anaesthetized rats. In conscious rats, RS-45041-190 had no effect on body core temperature or tail skin temperature (1 mg kg-1, s.c.) or on activity or rotarod performance (10 mg kg-1, i.p.). There were also no effects on barbiturate sleeping time in mice after doses of 1-10 mg kg-1, i.p. 5. RS-45041-190 (10 and 25 mg kg-1, i.p.) significantly increased food consumption in rats for up to 4 h after dosing, but unlike idazoxan (10 mg kg-1, i.p.) did not increase water consumption. RS-45041-190 is therefore a selective, high-affinity ligand at I2 imidazoline receptors and its hyperphagic effect may suggest a role for I2 imidazoline receptors in the modulation of appetite.However, in the absence of a selective agonist it is unclear whether this ligand is an agonist or an antagonist at I2 receptors.


