NS 2028Potent soluble guanylyl cyclase (sGC) inhibitor CAS# 204326-43-2 |
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 204326-43-2 | SDF | Download SDF |
| PubChem ID | 4551 | Appearance | Powder |
| Formula | C9H5BrN2O3 | M.Wt | 269.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in ethanol | ||
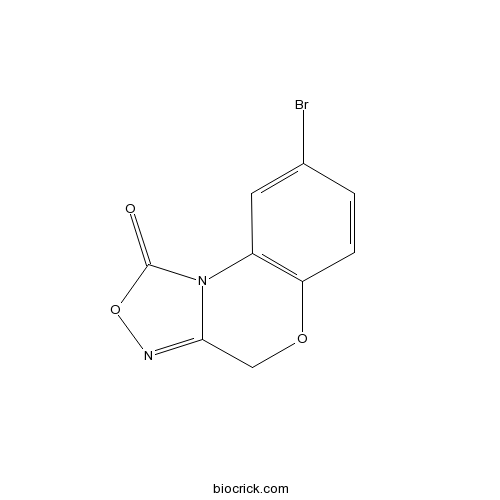
| Chemical Name | 8-bromo-4H-[1,2,4]oxadiazolo[3,4-c][1,4]benzoxazin-1-one | ||
| SMILES | C1C2=NOC(=O)N2C3=C(O1)C=CC(=C3)Br | ||
| Standard InChIKey | MUDRLQRJCGJJTB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H5BrN2O3/c10-5-1-2-7-6(3-5)12-8(4-14-7)11-15-9(12)13/h1-3H,4H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent soluble guanylyl cyclase (sGC) inhibitor (Ki = 8 nM). Blocks sGC activity in murine cerebellum induced by S-nitroso-glutathione and NMDA (IC50 values are 17 and 20 nM respectively). Inhibits VEGF-induced cGMP accumulation; abolishes VEGF-induced migration in postcapillary venular endothelial cells (CVEC). |

NS 2028 Dilution Calculator

NS 2028 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7168 mL | 18.5839 mL | 37.1678 mL | 74.3356 mL | 92.9195 mL |
| 5 mM | 0.7434 mL | 3.7168 mL | 7.4336 mL | 14.8671 mL | 18.5839 mL |
| 10 mM | 0.3717 mL | 1.8584 mL | 3.7168 mL | 7.4336 mL | 9.292 mL |
| 50 mM | 0.0743 mL | 0.3717 mL | 0.7434 mL | 1.4867 mL | 1.8584 mL |
| 100 mM | 0.0372 mL | 0.1858 mL | 0.3717 mL | 0.7434 mL | 0.9292 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RS 45041-190 hydrochloride
Catalog No.:BCC5682
CAS No.:204274-74-8
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
- Boc-Ser-OH.H2O
Catalog No.:BCC2599
CAS No.:204191-40-2
- Caesalmin E
Catalog No.:BCN7247
CAS No.:204185-91-1
- 4'-Hydroxy-2-O-methylanigorufone
Catalog No.:BCN7179
CAS No.:204134-70-3
- Anabasamine
Catalog No.:BCN2148
CAS No.:20410-87-1
- (2-Aminoethyl)phosphonic acid
Catalog No.:BCN1762
CAS No.:2041-14-7
- PD 176252
Catalog No.:BCC7426
CAS No.:204067-01-6
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
- Helioxanthin derivative 5-4-2
Catalog No.:BCC5414
CAS No.:203935-39-1
- Fmoc-ß-HoGlu(OtBu)-OH
Catalog No.:BCC3234
CAS No.:203854-49-3
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Fmoc-β-Homo-Phe-OH
Catalog No.:BCC2629
CAS No.:204384-69-0
- BCH
Catalog No.:BCC7993
CAS No.:20448-79-7
- Talnetant hydrochloride
Catalog No.:BCC1982
CAS No.:204519-66-4
- Epitaraxerol
Catalog No.:BCN4677
CAS No.:20460-33-7
- Olcegepant
Catalog No.:BCC1818
CAS No.:204697-65-4
- Fmoc-Lys(ivDde)-OH
Catalog No.:BCC3520
CAS No.:204777-78-6
- 8-O-Acetyltorilolone
Catalog No.:BCN7094
CAS No.:20482-21-7
- Procumbide
Catalog No.:BCN3932
CAS No.:20486-27-5
- Nardosinonediol
Catalog No.:BCN8118
CAS No.:20489-11-6
- Curzerenone
Catalog No.:BCN3009
CAS No.:20493-56-5
- 3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN1503
CAS No.:204935-85-3
- Talatisamine
Catalog No.:BCN5403
CAS No.:20501-56-8
The soluble guanylyl cyclase inhibitor NS-2028 reduces vascular endothelial growth factor-induced angiogenesis and permeability.[Pubmed:20032260]
Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R824-32.
Nitric oxide (NO) is known to promote vascular endothelial growth factor (VEGF)-stimulated permeability and angiogenesis. However, effector molecules that operate downstream of NO in this pathway remain poorly characterized. Herein, we determined the effect of soluble guanylyl cyclase (sGC) inhibition on VEGF responses in vitro and in vivo. Treatment of endothelial cells (EC) with VEGF stimulated eNOS phosphorylation and cGMP accumulation; pretreatment with the sGC inhibitor 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS-2028) blunted cGMP levels without affecting VEGF-receptor phosphorylation. Incubation of cells with NS-2028 blocked the mitogenic effects of VEGF. In addition, cells in which sGC was inhibited exhibited no migration and sprouting in response to VEGF. To study the mechanisms through which NS-2028 inhibits EC migration, we determined the effects of alterations in cGMP levels on p38 MAPK. Initially, we observed that inhibition of sGC attenuated VEGF-stimulated activation of p38. In contrast, the addition of 8-Br-cGMP to EC stimulated p38 phosphorylation. The addition of cGMP elevating agents (BAY 41-2272, DETA NO and YC-1) enhanced EC migration. To test whether sGC also mediated the angiogenic effects of VEGF in vivo, we used the rabbit cornea assay. Animals receiving NS-2028 orally displayed a reduced angiogenic response to VEGF. As increased vascular permeability occurs prior to new blood vessel formation, we determined the effect of NS-2028 in vascular leakage. Using a modified Miles assay, we observed that NS-2028 attenuated VEGF-induced permeability. Overall, we provide evidence that sGC mediates the angiogenic and permeability-promoting activities of VEGF, indicating the significance of sGC as a downstream effector of VEGF-triggered responses.
Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase.[Pubmed:9489619]
Br J Pharmacol. 1998 Jan;123(2):299-309.
1 The haeme-containing soluble guanylyl cyclase (alpha1beta1-heterodimer) is a major intracellular receptor and effector for nitric oxide (NO) and carbon monoxide (CO) and mediates many of their biological actions by increasing cyclic GMP. We have synthesized new oxadiazolo-benz-oxazins and have assessed their inhibitory actions on guanylyl cyclase activity in vitro, on the formation of cyclic GMP in cultured cells and on the NO-dependent relaxation of vascular and non-vascular smooth muscle. 2 Soluble guanylyl cyclase, purified to homogeneity from bovine lung, was inhibited by 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS 2028) in a concentration-dependent and irreversible manner (IC50 30 nM for basal and 200 nM for NO-stimulated enzyme activity). Evaluation of the inhibition kinetics according to Kitz & Wilson yielded a value of 8 nM for Ki, the equilibrium constant describing the initial reversible reaction between inhibitor and enzyme, and 0.2 min(-1) for the rate constant k3 of the subsequent irreversible inhibition. Inhibition was accompanied by a shift in the soret absorption maximum of the enzyme's haem cofactor from 430 to 390 nm. 3 S-nitroso-glutathione-enhanced soluble guanylyl cyclase activity in homogenates of mouse cerebellum was inhibited by NS 2028 (IC50 17 nM) and by 17 structural analogues in a similar manner, albeit with different potency, depending on the type of substitution at positions 1, 7 and 8 of the benzoxazin structure. Small electronegative ligands such as Br and Cl at position 7 or 8 increased and substitution of the oxygen at position 1 by -S-,- NH- or -CH2- decreased the inhibition. 4 In tissue slices prepared from mouse cerebellum, neuronal NO synthase-dependent activation of soluble guanylyl cyclase by the glutamate receptor agonist N-methyl-D-aspartate was inhibited by NS 2028 (IC50 20 nM) and by two of its analogues. Similarly, 3-morpholino-sydnonimine (SIN-1)-elicited formation of cyclic GMP in human cultured umbilical vein endothelial cells was inhibited by NS 2028 (IC50 30 nM). 5 In prostaglandin F2alpha-constricted, endothelium-intact porcine coronary arteries NS 2028 elicited a concentration-dependent increase (65%) in contractile tone (EC50 170 nM), which was abolished by removal of the endothelium. NS 2028 (1 microM) suppressed the relaxant response to nitroglycerin from 88.3+/-2.1 to 26.8+/-6.4% and induced a 9 fold rightward shift (EC50 15 microM) of the concentration-relaxation response curve to nitroglycerin. It abolished the relaxation to sodium nitroprusside (1 microM), but did not affect the vasorelaxation to the KATP channel opener cromakalim. Approximately 50% of the relaxant response to sodium nitroprusside was recovered after 2 h washout of NS 2028. 6 In phenylephrine-preconstricted, endothelium-denuded aorta of the rabbit NS 2028 (1 microM) did not affect relaxant responses to atrial natriuretic factor, an activator of particulate guanylyl cyclase, or forskolin, an activator of adenylyl cyclase. 7 NO-dependent relaxant responses in non-vascular smooth muscle were also inhibited by NS 2028. The nitroglycerin-induced relaxation of guinea-pig trachea preconstricted by histamine was fully inhibited by NS 2028 (1 microM), whereas the relaxations to terbutaline, theophylline and vasoactive intestinal polypeptide (VIP) were not affected. The relaxant responses to electrical field stimulation of non-adrenergic, non-cholinergic nerves in the same tissue were attenuated by 50% in the presence of NS 2028 (1 microM). 8 NS 2028 and its analogues, one of which is the previously characterized 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ), appear to be potent and specific inhibitors of soluble guanylyl cyclase present in various cell types. Oxidation and/or a change in the coordination of the haeme-iron of guanylyl cyclase is a likely inhibitory mechanism.
Synthesis and biological evaluation of oxadiazole derivatives as inhibitors of soluble guanylyl cyclase.[Pubmed:20036129]
Bioorg Med Chem. 2010 Feb;18(3):1288-96.
Soluble guanylyl cyclase (sGC) is an ubiquitously expressed enzyme that generates the second messenger cGMP and hence, leads to a number of physiological responses including vasodilation, inhibition of platelet aggregation and neurotransmission. Whilst many activating and stimulating modulators of sGC were identified and studied in recent years, only two selective inhibitors are known: ODQ and NS 2028. Furthermore, a synthetic approach to these inhibitors has not been reported yet. Herein, we describe a novel and efficient synthesis of these inhibitors, as well as the preparation of three different classes of NS 2028 analogues. Biological evaluation of this library using rat aortic smooth muscle cells revealed four new compounds with good to moderate sGC inhibitory activity. Our experiments underline the major importance of the oxadiazole ring in ODQ and NS 2028 for the efficiency of this class of inhibitors.


