Sagittatoside ACAS# 118525-35-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118525-35-2 | SDF | Download SDF |
| PubChem ID | 13916054 | Appearance | Yellow powder |
| Formula | C33H40O15 | M.Wt | 676.67 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Icariin-A | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
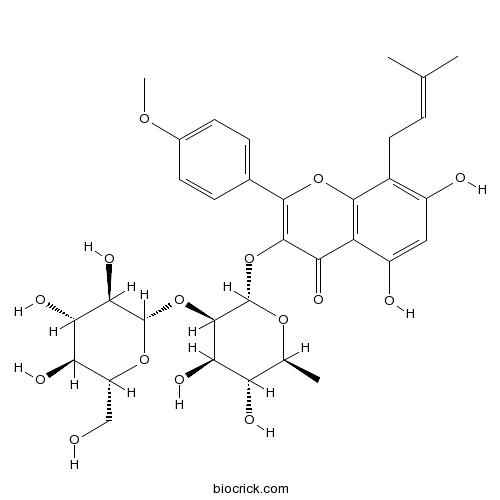
| Chemical Name | 3-[(2S,3R,4R,5R,6S)-4,5-dihydroxy-6-methyl-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2=C(OC3=C(C2=O)C(=CC(=C3CC=C(C)C)O)O)C4=CC=C(C=C4)OC)OC5C(C(C(C(O5)CO)O)O)O)O)O | ||
| Standard InChIKey | COHHGQPQHHUMDG-WVQJJEIESA-N | ||
| Standard InChI | InChI=1S/C33H40O15/c1-13(2)5-10-17-18(35)11-19(36)21-24(39)30(28(46-29(17)21)15-6-8-16(43-4)9-7-15)47-33-31(26(41)22(37)14(3)44-33)48-32-27(42)25(40)23(38)20(12-34)45-32/h5-9,11,14,20,22-23,25-27,31-38,40-42H,10,12H2,1-4H3/t14-,20+,22-,23+,25-,26+,27+,31+,32-,33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sagittatoside A selectively activates estrogen response element (ERE)-luciferase activity via ERα, and sagittatoside A and icariin induces ERα phosphorylation at serine 118 residue. |
| Targets | NF-kB | Estrogen receptor | Progestogen receptor |
| In vitro | Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERα) and stimulate ER-dependent osteoblastic functions in UMR-106 cells.[Pubmed: 24607839]J Steroid Biochem Mol Biol. 2014 Sep;143:141-51.Total flavonoids in Herba epimedii (HEP) have been demonstrated to protect against bone loss and bone deterioration associated with estrogen deficiency without exerting any uterotrophic effects. However, it is unclear how flavonoids in HEP exert their protective effects on bone and if different flavonoids exert estrogenic actions in bone cells via similar mechanism of actions. |
| Structure Identification | J Sep Sci. 2009 Jan;32(2):275-81.Effect of stability of internal standard on quantification of 15 flavonoids in Epimedium using CZE.[Pubmed: 19101945]
|

Sagittatoside A Dilution Calculator

Sagittatoside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4778 mL | 7.3891 mL | 14.7783 mL | 29.5565 mL | 36.9456 mL |
| 5 mM | 0.2956 mL | 1.4778 mL | 2.9557 mL | 5.9113 mL | 7.3891 mL |
| 10 mM | 0.1478 mL | 0.7389 mL | 1.4778 mL | 2.9557 mL | 3.6946 mL |
| 50 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5911 mL | 0.7389 mL |
| 100 mM | 0.0148 mL | 0.0739 mL | 0.1478 mL | 0.2956 mL | 0.3695 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sagittatoside A is a natural compound isolated from traditional Chinese herb Yinyanghuo (Herba Epimdii).
- DPPE fumarate
Catalog No.:BCC5669
CAS No.:1185241-83-1
- LP 12 hydrochloride
Catalog No.:BCC7517
CAS No.:1185136-22-4
- Myelin Basic Protein (87-99)
Catalog No.:BCC1028
CAS No.:118506-26-6
- TRIS hydrochloride
Catalog No.:BCC7589
CAS No.:1185-53-1
- 5,7-Dichlorokynurenic acid sodium salt
Catalog No.:BCC7758
CAS No.:1184986-70-6
- Fmoc-D-Tyr(tBu)-OH
Catalog No.:BCC3569
CAS No.:118488-18-9
- Cyclo(L-Phe-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3989
CAS No.:118477-06-8
- Arcyriaflavin A
Catalog No.:BCC7370
CAS No.:118458-54-1
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- MK 886
Catalog No.:BCC7017
CAS No.:118414-82-7
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- Sagittatoside B
Catalog No.:BCN2357
CAS No.:118525-36-3
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
- Icaritin
Catalog No.:BCN5352
CAS No.:118525-40-9
- Baohuoside V
Catalog No.:BCN2887
CAS No.:118544-18-6
- H-Orn(2-Cl-Z)-OH
Catalog No.:BCC3002
CAS No.:118553-99-4
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERalpha) and stimulate ER-dependent osteoblastic functions in UMR-106 cells.[Pubmed:24607839]
J Steroid Biochem Mol Biol. 2014 Sep;143:141-51.
Total flavonoids in Herba epimedii (HEP) have been demonstrated to protect against bone loss and bone deterioration associated with estrogen deficiency without exerting any uterotrophic effects. However, it is unclear how flavonoids in HEP exert their protective effects on bone and if different flavonoids exert estrogenic actions in bone cells via similar mechanism of actions. The present study aims to investigate the bone anabolic effects of four major flavonoids isolated from HEP, namely icariin, baohuoside-I, epimedin B and Sagittatoside A as well as the mechanism involved in mediating their estrogenic actions in rat osteoblastic-like UMR-106 cells. All tested compounds significantly stimulated the cell proliferation rate, alkaline phosphate (ALP) activity and osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B ligand (RANKL) mRNA expression in UMR-106 cells and their effects could be abolished by co-incubation with 10(-6)M ICI 182,780. None of the flavonoids exhibited binding affinities toward ERalpha and ERbeta. However, Sagittatoside A selectively activated estrogen response element (ERE)-luciferase activity via ERalpha. In addition, icariin and Sagittatoside A induced ERalpha phosphorylation at serine 118 residue. Taken together, our results indicated that all four flavonoids from HEP stimulated ER-dependent osteoblastic functions in UMR-106 cells, but only two of them appeared to exert their actions by ligand-independent activation of ERalpha. Our study provides evidence to support the hypothesis that the estrogen-like protective effects on bone by flavonoids are mediated via mechanisms that are distinct from the classical actions of estrogen.
Effect of stability of internal standard on quantification of 15 flavonoids in Epimedium using CZE.[Pubmed:19101945]
J Sep Sci. 2009 Jan;32(2):275-81.
A CZE method was developed for the simultaneous determination of 15 flavonoids, including epimedin B, epimedin A, hexandraside F, epimedin C, icariin, sagittatoside B, Sagittatoside A, hexandraside E, 2''-O-rhamnosyl icariside II, baohuoside VII, baohuoside I, caohuoside C, epimedoside C, baohuoside II, and kaempferol-3-O-rhamnoside, in different species of Epimedium, and the effect of stability of internal standard (IS) on quantification was also investigated. As a result, rutin was not available for use as an IS because of its unstable property in sample solution, which suggested that the stability of IS both in standards and sample solution should be considered for the analysis. Using stable daidzein as IS, the analysis was performed within 35 min by using 50 mM borax buffer containing 20% ACN as a modifier (pH 10.0), while separation voltage was 25 kV and temperature was at 30 degrees C. The method was validated to be accurate, simple, and repeatable, and was successfully applied to the analysis of 36 samples from 17 species of Epimedium.


