MK 886Inhibitor of 5-lipoxygenase-activating protein (FLAP) CAS# 118414-82-7 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CCT241533 hydrochloride
Catalog No.:BCC1463
CAS No.:1431697-96-9
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118414-82-7 | SDF | Download SDF |
| PubChem ID | 105049 | Appearance | Powder |
| Formula | C27H34ClNO2S | M.Wt | 472.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-663,536 | ||
| Solubility | Soluble to 5 mM in ethanol and to 100 mM in DMSO | ||
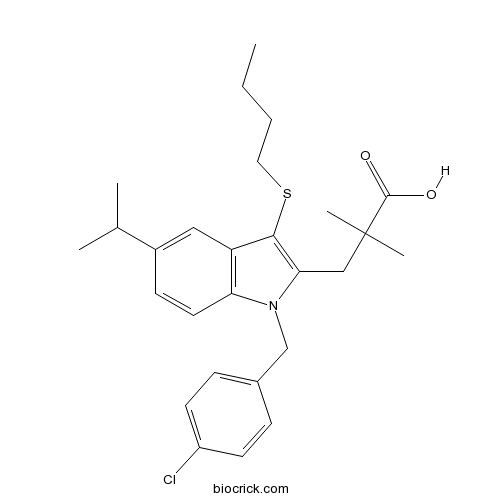
| Chemical Name | 3-[3-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-propan-2-ylindol-2-yl]-2,2-dimethylpropanoic acid | ||
| SMILES | CCCCSC1=C(N(C2=C1C=C(C=C2)C(C)C)CC3=CC=C(C=C3)Cl)CC(C)(C)C(=O)O | ||
| Standard InChIKey | VFMGWQLOCZBFCK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H34ClNO2S/c1-6-7-14-32-25-22-15-20(18(2)3)10-13-23(22)29(17-19-8-11-21(28)12-9-19)24(25)16-27(4,5)26(30)31/h8-13,15,18H,6-7,14,16-17H2,1-5H3,(H,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An inhibitor of leukotriene biosynthesis (IC50 = 3 nM in human polymorphonuclear leukocytes). Acts by inhibiting 5-lipoxygenase-activating protein (FLAP) (IC50 = 30 nM for inhibition of [125I]-L-691,678 photoaffinity labelling). Also moderately potent PPARα antagonist (IC50 = 0.5-1 μM). Orally active in vivo. |

MK 886 Dilution Calculator

MK 886 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1183 mL | 10.5914 mL | 21.1829 mL | 42.3657 mL | 52.9571 mL |
| 5 mM | 0.4237 mL | 2.1183 mL | 4.2366 mL | 8.4731 mL | 10.5914 mL |
| 10 mM | 0.2118 mL | 1.0591 mL | 2.1183 mL | 4.2366 mL | 5.2957 mL |
| 50 mM | 0.0424 mL | 0.2118 mL | 0.4237 mL | 0.8473 mL | 1.0591 mL |
| 100 mM | 0.0212 mL | 0.1059 mL | 0.2118 mL | 0.4237 mL | 0.5296 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK886 is a potent 5-lipoxygenase activating protein inhibitor (FLAP) also a non-competitive inhibitor of PPAR alpha. a potent inhibitor of leukotriene (LT) biosynthesis in intact human polymorphonuclear leukocytes with IC 50 of 2.5 nM. Block the synthessis of leukotrien intact activate leukocyte. target: FLAP, LT IC50: 2.5 nM ( leukotriene ,LT ) In vitro: MK886 inhibit PPAR alpha by a non-competitive mechanism as shown by its effects on the binding of arachidonic acid to PPAR alpha protein. The expression of keratin-1 is reduced by MK886 in a culture of mouse primary keratinocytes. MK886 functioning as a non-competitive inhibitor of PPAR alpha, but may also indicate that PPAR alpha is not directly involved in MK886-induced apoptosis. In vivo: L-663,536 inhibit A23187-induced LTB4 formation by rat peripheral blood and elicited PMN. The compound inhibited leukotriene biosynthesis in vivo in a rat pleurisy model (ED50, 0.2 mg/kg p.o.), an inflamed rat paw model (ED50, 0.8 mg/kg)
References:
[1]. [1] Kehrer JP et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochem J. 2001 Jun 15.
[2]. [2] Gillard J et al. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456-64.
[3]. [3] Dixon RA et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature, 1990 Jan 18, 343(6255):282-4. http://www.ncbi.nlm.nih.gov/pubmed/2300173
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- Trimethylamine oxide
Catalog No.:BCN1819
CAS No.:1184-78-7
- Australine
Catalog No.:BCN2053
CAS No.:118396-02-4
- Licoricesaponin A3
Catalog No.:BCN7905
CAS No.:118325-22-7
- Tazarotene
Catalog No.:BCC2540
CAS No.:118292-40-3
- 6-O-Acetylscandoside
Catalog No.:BCN8320
CAS No.:118292-15-2
- AF-DX 384
Catalog No.:BCC7024
CAS No.:118290-26-9
- Lafutidine
Catalog No.:BCC4544
CAS No.:118288-08-7
- Isodorsmanin A
Catalog No.:BCN6460
CAS No.:118266-99-2
- 1,4-Dicaffeoylquinic acid
Catalog No.:BCN5912
CAS No.:1182-34-9
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- Arcyriaflavin A
Catalog No.:BCC7370
CAS No.:118458-54-1
- Cyclo(L-Phe-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3989
CAS No.:118477-06-8
- Fmoc-D-Tyr(tBu)-OH
Catalog No.:BCC3569
CAS No.:118488-18-9
- 5,7-Dichlorokynurenic acid sodium salt
Catalog No.:BCC7758
CAS No.:1184986-70-6
- TRIS hydrochloride
Catalog No.:BCC7589
CAS No.:1185-53-1
- Myelin Basic Protein (87-99)
Catalog No.:BCC1028
CAS No.:118506-26-6
- LP 12 hydrochloride
Catalog No.:BCC7517
CAS No.:1185136-22-4
- DPPE fumarate
Catalog No.:BCC5669
CAS No.:1185241-83-1
- Sagittatoside A
Catalog No.:BCN2285
CAS No.:118525-35-2
- Sagittatoside B
Catalog No.:BCN2357
CAS No.:118525-36-3
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
Potentiation of hypericin-mediated photodynamic therapy cytotoxicity by MK-886: focus on ABC transporters, GDF-15 and redox status.[Pubmed:26003114]
Photodiagnosis Photodyn Ther. 2015 Sep;12(3):490-503.
BACKGROUND: Pretreatment with 5-LOX pathway inhibitor MK-886 potentiates cytotoxic effects of photodynamic therapy mediated by natural photosensitizer, hypericin. In this study, we focused on elucidating mechanisms beyond the increased efficacy of combined treatment. METHODS: Metabolic activity/viability, caspase-3 activation/mitochondrial membrane potential dissipation, intracellular hypericin level, glutathione level and redox status (NAD(P)H/oxidized flavins ratio) analyses, as well as drug efflux assays, were performed by flow cytometry. Changes in protein expression of ATP-binding cassette transporters, GDF-15 and other selected proteins were evaluated by Western blotting. Silencing of gdf-15 was carried out to verify its role in response to treatment. RESULTS: MK-886 pretreatment led to a concentration-dependent increase in intracellular hypericin content, accompanied by changes in ATP-binding cassette transporters levels and efflux efficiency. Intracellular accumulation of cytokine GDF-15 correlated with increased cell death markers; however, the impact of gdf-15 silencing on the evaluated markers was negligible. A marked decrease in the glutathione level of a majority of cells was observed after more toxic combination treatment. CONCLUSION: The significant increase in cell death markers after combination treatment confirms the potentiating effect of MK-886 on hypericin-mediated photodynamic therapy in HT-29 and MCF-7 cells. Although BCRP downregulation was not confirmed as leading mechanism responsible for elevated levels of hypericin content, changes in expression and efflux activity of ABC transporters caused by MK-886 suggest its potential in combination treatment with drugs that are substrates of these transporters, predominantly MRP1. However, complex cellular response to MK-886 pretreatment needs to be considered and further elucidated.
MK-886, an inhibitor of the 5-lipoxygenase-activating protein, inhibits cyclooxygenase-1 activity and suppresses platelet aggregation.[Pubmed:19239910]
Eur J Pharmacol. 2009 Apr 17;608(1-3):84-90.
MK-886, an inhibitor of the 5-lipoxygenase-activating protein (FLAP), potently suppresses leukotriene biosynthesis in intact cells and is frequently used to define a role of the 5-lipoxygenase (EC 1.13.11.34) pathway in cellular or animal models of inflammation, allergy, cancer, and cardiovascular disease. Here we show that MK-886 also interferes with the activities of cyclooxygenases (COX, EC 1.14.99.1). MK-886 inhibited isolated COX-1 (IC(50)=8 microM) and blocked the formation of the COX-1-derived products 12(S)-hydroxy-5-cis-8,10-trans-heptadecatrienoic acid (12-HHT) and thromboxane B(2) in washed human platelets in response to collagen as well as from exogenous arachidonic acid (IC(50)=13-15 microM). Isolated COX-2 was less affected (IC(50)=58 microM), and in A549 cells, MK-886 (33 microM) failed to suppress COX-2-dependent 6-keto-prostaglandin (PG)F(1alpha) formation. The distinct susceptibility of MK-886 towards COX-1 and -2 is apparent in automated molecular docking studies that indicate a preferred binding of MK-886 to COX-1 into the active site. MK-886 (10 microM) inhibited COX-1-mediated platelet aggregation induced by collagen or arachidonic acid whereas thrombin- or U-46619-induced (COX-independent) aggregation was not affected. Since leukotrienes and prostaglandins share (patho)physiological properties in the development and regulation of carcinogenesis, inflammation, and vascular functions, caution should be used when interpreting data where MK-886 is used as tool to determine the involvement of FLAP and/or the 5-lipoxygenase pathway in respective experimental models.
Mechanisms involved in the cell cycle and apoptosis of HT-29 cells pre-treated with MK-886 prior to photodynamic therapy with hypericin.[Pubmed:18771933]
J Photochem Photobiol B. 2008 Nov 13;93(2):108-18.
In our previous study we have proved that colon cancer cells HT-29 pre-treated with specific 5-lipoxygenase inhibitor MK-886 became more susceptible to photodynamic therapy (PDT) with hypericin and we also found that this mutual combination induced cell cycle arrest and stimulated onset of apoptosis (Kleban et al., 2007. J. Photochem. Photobiol. B 84, 2). To further explain events associated with MK-886 mediated sensitization of tumor cells toward PDT with hypericin, more detailed study of signaling pathways leading to increase in apoptosis as well as cell cycle perturbations was performed and is presented herein. Intensive accumulation of HT-29 cells in G0/G1 phase of cell cycle led to expression analyses of several G0/G1 checkpoint molecules (cyclin A, cyclin E, cdk-2, pRb). Similarly, accumulation of apoptotic cells invoked analyses of key molecules involved in apoptotic signaling (caspase-3, -8, -9; PARP; Lamin B; Mcl-1; Bax) by Western blotting and caspase activity assay. Long term survival of cells was examined by clonogenicity test. As the effect of PDT is mediated by ROS production, levels of hydrogen peroxides and superoxide anion were monitored by flow cytometric analyses. In addition, an impact of MK-886 on LTB4 production and expression of 5-LOX was monitored. Massive G0/G1 arrest in the cell cycle accompanied by increase in cyclin E level and decrease/absention of cyclin A, cdk-2 and pRb expression indicated incapability for G1/S transition. Minimal changes in cleavage of procaspases observed in cells treated with non-toxic concentrations of either agent alone or their mutual combination were not quite in line with their activity (caspase-3, -8, -9) which was significantly increased mainly in combinations. Treatment with non-toxic concentration of MK-886 had minimal influence over ROS production compared to control cells. In contrast, hypericin alone markedly increased the level of ROS, but no additional effect of MK-886 pre-treatment was detected. Further analyses of particular ROS groups unveiled an impact of increasing MK-886 concentration on superoxide accumulation accompanied with depletion of hydrogen peroxide level within the cells. The clonogenicity test revealed disruption of colony formation after mutual combination of both agents as compared to MK-886 or PDT alone. In conclusion, we presume that stimulation of apoptosis in our experimental model was accomplished preferentially through the mitochondrial pathway, although caspase-8 activation was also noticed. Interestingly, pre-treatment with MK-886 modulated distribution of ROS production in mutual combination with PDT.
Effect of MK-886 on Ca2+ level and viability in PC3 human prostate cancer cells.[Pubmed:19371256]
Basic Clin Pharmacol Toxicol. 2009 Jun;104(6):441-7.
3-[1-(p-chlorobenzyl)-5-(isopropyl)-3-tert-butylthioindol-2-yl]-2, 2-dimethylpropanoic acid (MK-886) is widely used for inhibition of leucotriene synthesis in in vitro studies, however, many of its other effects have been reported. The present study investigated the effect of MK-886 on cytosolic-free Ca(2+) concentrations ([Ca(2+)](i)) and viability in human PC3 prostate cancer cells. [Ca(2+)](i) in suspended cells was measured by using fura-2. MK-886 at concentrations of 1 microM and above increased [Ca(2+)](i) in a concentration-dependent manner with an EC(50) value of 20 microM. The Ca(2+) signal was reduced partly by removing extracellular Ca(2+). MK-886 evoked Mn(2+) quenching of fura-2 fluorescence, implicating Ca(2+) entry. MK-886-induced Ca(2+) influx was inhibited by store-operated Ca(2+) entry inhibitors nifedipine, econazole and SKF96365. In Ca(2+)-free medium, after pre-treatment with 10 microM MK-886, 1 microM thapsigargin (an endoplasmic reticulum Ca(2+) pump inhibitor)-induced [Ca(2+)](i) rises were abolished; and conversely, thapsigargin pre-treatment abolished MK-886-induced [Ca(2+)](i) rises. Inhibition of phospholipase C with U73122 did not alter MK-886-induced [Ca(2+)](i) rises. MK-886 at concentrations of 1-100 microM concentration-dependently decreased cell viability with an IC(50) value of 60 microM. The cytotoxic effect of MK-886 was not inhibited by pre-chelating cytosolic Ca(2+) with BAPTA/AM. Together, in PC3 cells, MK-886 induced [Ca(2+)](i) rises by causing phospholipase C-independent Ca(2+) release from the endoplasmic reticulum; and Ca(2+) influx via store-operated Ca(2+) channels. Independently, MK-886 was cytotoxic to cells in a Ca(2+)-independent manner.
Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886.[Pubmed:11389700]
Biochem J. 2001 Jun 15;356(Pt 3):899-906.
Although MK886 was originally identified as an inhibitor of 5-lipoxygenase activating protein (FLAP), recent data demonstrate that this activity does not underlie its ability to induce apoptosis [Datta, Biswal and Kehrer (1999) Biochem. J. 340, 371--375]. Since FLAP is a fatty-acid binding protein, it is conceivable that MK886 may affect other such proteins. A family of nuclear receptors that are activated by fatty acids and their metabolites, the peroxisome-proliferator-activated receptors (PPARs), have been implicated in apoptosis and may represent a target for MK886. The ability of MK886 to inhibit PPAR-alpha, -beta and -gamma activity was assessed using reporter assay systems (peroxisome-proliferator response element--luciferase). Using a transient transfection system in monkey kidney fibroblast CV-1 cells, mouse keratinocyte 308 cells and human lung adenocarcinoma A549 cells, 10--20 microM MK886 inhibited Wy14,643 activation of PPAR alpha by approximately 80%. Similar inhibition of PPAR alpha by MK886 was observed with a stable transfection reporter system in CV-1 cells. Only minimal inhibitory effects were seen on PPAR beta and PPAR gamma. MK886 inhibited PPAR alpha by a non-competitive mechanism as shown by its effects on the binding of arachidonic acid to PPAR alpha protein, and a dose-response study using a transient transfection reporter assay in COS-1 cells. An assay assessing PPAR ligand-receptor interactions showed that MK886 prevents the conformational change necessary for active-complex formation. The expression of keratin-1, a protein encoded by a PPAR alpha-responsive gene, was reduced by MK886 in a culture of mouse primary keratinocytes, suggesting that PPAR inhibition has functional consequences in normal cells. Although Jurkat cells express all PPAR isoforms, various PPAR alpha and PPAR gamma agonists were unable to prevent MK886-induced apoptosis. This is consistent with MK886 functioning as a non-competitive inhibitor of PPAR alpha, but may also indicate that PPAR alpha is not directly involved in MK886-induced apoptosis. Although numerous PPAR activators have been identified, the results show that MK886 can inhibit PPAR alpha, making it the first compound identified to have such an effect.
5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors.[Pubmed:1538707]
Mol Pharmacol. 1992 Feb;41(2):267-72.
An 18-kDa leukocyte membrane protein, termed 5-lipoxygenase-activating protein (FLAP), has recently been shown to be the target of two structurally distinct classes of leukotriene biosynthesis inhibitors. These classes of inhibitors are based on indole and quinoline structures and are represented by MK-886 and L-674,573, respectively. A novel class of hybrid structure based on the indole and quinoline classes of inhibitors, termed quindoles, has recently been developed. These compounds, exemplified by L-689,037, are potent inhibitors of leukotriene biosynthesis, both in vitro and in vivo. In the present study, we have developed and characterized a potent radioiodinated photoaffinity analogue of L-689,037, termed [125I]L-691,678. This compound was used in immunoprecipitation studies with FLAP antisera to show that the quindole series of leukotriene biosynthesis inhibitors interact directly with FLAP. In addition, we show that MK-886, L-674,573, and L-689,037 specifically compete, in a concentration-dependent manner, with both [125I]L-691,678 and [125I]L-669,083, a photoaffinity analogue of MK-886, for binding to FLAP. These results suggest that these three classes of leukotriene biosynthesis inhibitors share a common binding site on FLAP, providing further evidence that FLAP represents a suitable target for structurally diverse classes of leukotriene biosynthesis inhibitors.
Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis.[Pubmed:2300173]
Nature. 1990 Jan 18;343(6255):282-4.
Leukotrienes, the biologically active metabolites of arachidonic acid, have been implicated in a variety of inflammatory responses, including asthma, arthritis and psoriasis. Recently a compound, MK-886, has been described that blocks the synthesis of leukotrienes in intact activated leukocytes, but has little or no effect on enzymes involved in leukotriene synthesis, including 5-lipoxygenase, in cell-free systems. A membrane protein with a high affinity for MK-886 and possibly representing the cellular target for MK-886 has been isolated from rat and human leukocytes. Here, we report the isolation of a complementary DNA clone encoding the MK-886-binding protein. We also demonstrate that the expression of both the MK-886-binding protein and 5-lipoxygenase is necessary for leukotriene synthesis in intact cells. Because the MK-886-binding protein seems to play a part in activating this enzyme in cells, it is termed the five-lipoxygenase activating protein (FLAP).
L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor.[Pubmed:2548691]
Can J Physiol Pharmacol. 1989 May;67(5):456-64.
L-663,536 (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2, 2-dimethylpropanoic acid) is a potent inhibitor of leukotriene (LT) biosynthesis in intact human polymorphonuclear leukocytes (PMN) (IC50, 2.5 nM). Similarly, L-663,536 inhibited A23187-induced LTB4 formation by rat peripheral blood and elicited PMN. At concentrations where inhibition of leukotriene biosynthesis occurred in human whole blood (1.1 microM), no effect was seen on cyclooxygenase or 12-lipoxygenase, an effect also observed in washed human platelets. The compound had no effect on rat or porcine 5-lipoxygenase indicating that L-663,536 is not a direct 5-lipoxygenase inhibitor. When administered in vivo L-663,536 was a potent inhibitor of antigen-induced dyspnea in inbred rats pretreated with methysergide (ED50, 0.036 mg/kg p.o.) and of Ascaris-induced bronchoconstriction in squirrel monkeys (1 mg/kg p.o.). The compound inhibited leukotriene biosynthesis in vivo in a rat pleurisy model (ED50, 0.2 mg/kg p.o.), an inflamed rat paw model (ED50, 0.8 mg/kg), a model of leukotriene excretion in rat bile following antigen provocation, and a model in the guinea-pig ear where leukotriene synthesis was induced by topical challenge with ionophore A23187 (ED50, 2.5 mg/kg p.o. and 0.6 micrograms topically). The results indicate that L-663,536 is a potent inhibitor of leukotriene biosynthesis both in vitro and in vivo indicating that the compound is suitable for studying the role of leukotrienes in a variety of pathological situations.


