LafutidineCAS# 118288-08-7 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118288-08-7 | SDF | Download SDF |
| PubChem ID | 5282136 | Appearance | Powder |
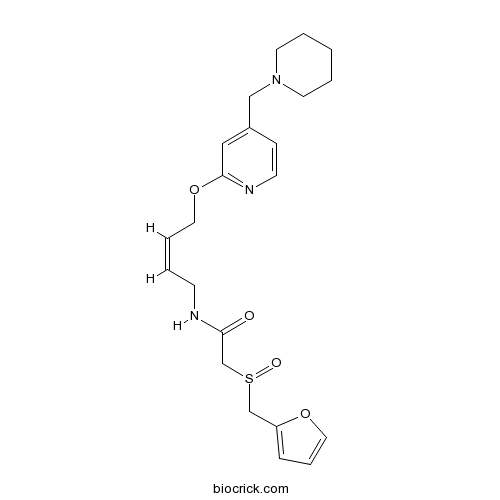
| Formula | C22H29N3O4S | M.Wt | 431.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (115.86 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(furan-2-ylmethylsulfinyl)-N-[(Z)-4-[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxybut-2-enyl]acetamide | ||
| SMILES | C1CCN(CC1)CC2=CC(=NC=C2)OCC=CCNC(=O)CS(=O)CC3=CC=CO3 | ||
| Standard InChIKey | KMZQAVXSMUKBPD-DJWKRKHSSA-N | ||
| Standard InChI | InChI=1S/C22H29N3O4S/c26-21(18-30(27)17-20-7-6-14-28-20)23-9-2-5-13-29-22-15-19(8-10-24-22)16-25-11-3-1-4-12-25/h2,5-8,10,14-15H,1,3-4,9,11-13,16-18H2,(H,23,26)/b5-2- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lafutidine Dilution Calculator

Lafutidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3172 mL | 11.5861 mL | 23.1723 mL | 46.3446 mL | 57.9307 mL |
| 5 mM | 0.4634 mL | 2.3172 mL | 4.6345 mL | 9.2689 mL | 11.5861 mL |
| 10 mM | 0.2317 mL | 1.1586 mL | 2.3172 mL | 4.6345 mL | 5.7931 mL |
| 50 mM | 0.0463 mL | 0.2317 mL | 0.4634 mL | 0.9269 mL | 1.1586 mL |
| 100 mM | 0.0232 mL | 0.1159 mL | 0.2317 mL | 0.4634 mL | 0.5793 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lafutidine, a newly developed histamine H(2)-receptor antagonist, inhibits gastric acid secretion.
- Isodorsmanin A
Catalog No.:BCN6460
CAS No.:118266-99-2
- 1,4-Dicaffeoylquinic acid
Catalog No.:BCN5912
CAS No.:1182-34-9
- Soyasaponin Ab
Catalog No.:BCN2896
CAS No.:118194-13-1
- EMD638683
Catalog No.:BCC1551
CAS No.:1181770-72-8
- 2-Picenecarboxylic acid
Catalog No.:BCN3063
CAS No.:118172-80-8
- 6-Oxo-23-norpristimerol
Catalog No.:BCN8054
CAS No.:118172-79-5
- 6''-O-Acetylastragalin
Catalog No.:BCN6058
CAS No.:118169-27-0
- Volvaltrate B
Catalog No.:BCN6736
CAS No.:1181224-13-4
- [Phe8Ψ(CH-NH)-Arg9]-Bradykinin
Catalog No.:BCC5995
CAS No.:118122-39-7
- Schisanwilsonin I
Catalog No.:BCN5548
CAS No.:1181216-84-1
- Schisanwilsonin H
Catalog No.:BCN3315
CAS No.:1181216-83-0
- Karounidiol
Catalog No.:BCN2704
CAS No.:118117-31-0
- AF-DX 384
Catalog No.:BCC7024
CAS No.:118290-26-9
- 6-O-Acetylscandoside
Catalog No.:BCN8320
CAS No.:118292-15-2
- Tazarotene
Catalog No.:BCC2540
CAS No.:118292-40-3
- Licoricesaponin A3
Catalog No.:BCN7905
CAS No.:118325-22-7
- Australine
Catalog No.:BCN2053
CAS No.:118396-02-4
- Trimethylamine oxide
Catalog No.:BCN1819
CAS No.:1184-78-7
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- MK 886
Catalog No.:BCC7017
CAS No.:118414-82-7
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- Arcyriaflavin A
Catalog No.:BCC7370
CAS No.:118458-54-1
Randomized study of lafutidine vs lansoprazole in patients with mild gastroesophageal reflux disease.[Pubmed:27340360]
World J Gastroenterol. 2016 Jun 21;22(23):5430-5.
AIM: To compare the clinical efficacy of the second-generation H2RA Lafutidine with that of lansoprazole in Japanese patients with mild gastroesophageal reflux disease (GERD). METHODS: Patients with symptoms of GERD and a diagnosis of grade A reflux esophagitis (according to the Los Angeles classification) were randomized to receive Lafutidine (10 mg, twice daily) or lansoprazole (30 mg, once daily) for an initial 8 wk, followed by maintenance treatment comprising half-doses of the assigned drug for 24 wk. The primary endpoint was the frequency and severity of heartburn during initial and maintenance treatment. The secondary endpoints were the sum score of questions 2 and 3 in the Gastrointestinal Symptom Rating Scale (GSRS), and the satisfaction score. RESULTS: Between April 2012 and March 2013, a total of 53 patients were enrolled, of whom 24 and 29 received Lafutidine and lansoprazole, respectively. After 8 wk, the frequency and severity of heartburn was significantly reduced in both groups. However, Lafutidine was significantly inferior to lansoprazole with regard to the severity of heartburn during initial and maintenance treatment (P = 0.016). The sum score of questions 2 and 3 in the GSRS, and satisfaction scores were also significantly worse in the Lafutidine group than the lansoprazole group (P = 0.0068 and P = 0.0048, respectively). CONCLUSION: The clinical efficacy of Lafutidine was inferior to that of lansoprazole, even in Japanese patients with mild GERD.
Studies of DNA-binding properties of lafutidine as adjuvant anticancer agent to calf thymus DNA using multi-spectroscopic approaches, NMR relaxation data, molecular docking and dynamical simulation.[Pubmed:28235605]
Int J Biol Macromol. 2017 Jun;99:79-87.
The interactions between Lafutidine (LAF) and calf thymus DNA (ctDNA) have been investigated both experimentally and theoretically. UV-vis absorption studies confirmed that LAF binds to ctDNA through non-covalent interactions. Fluorescence quenching and time-resolved fluorescence spectroscopy studies showed that the binding of LAF with ctDNA occurred through static quenching mechanism, resulting in the formation of a LAF-ctDNA complex. The binding constants (K) of the complex were found to be around 10(3)M(-1) via NMR relaxation rates and fluorescence data, and the calculated thermodynamic parameters indicated that hydrogen bonds and van der Waals forces played major roles in the binding of LAF to ctDNA. The changes in CD spectra indicated that LAF induced a slight perturbation on the base stacking and helicity of B-DNA. A comparative study of the LAF-ctDNA complex with respect to potassium iodide quenching experiments and competition displacement assays with ethidium bromide, acridine orange, and Hoechst 33258 probes suggested that LAF interacted with ctDNA by minor groove mode. Molecular docking analysis further supported the minor groove binding. Molecular dynamics simulation indicated that LAF depart from the C-G region of DNA, but it can steadily bind with the middle part of DNA composed by A-T base pairs.
Pharmacokinetic Properties of Oral Lafutidine Tablets and the Effect of Food on its Pharmacokinetics in Healthy Chinese Subjects.[Pubmed:27444313]
Adv Ther. 2016 Oct;33(10):1704-1714.
INTRODUCTION: The aim of this study was to evaluate the pharmacokinetics (PK) of single and multiple doses of oral Lafutidine tablets and the effect of food on the PK properties in healthy Chinese subjects. The tolerability and the effect of gender on the PK properties were also evaluated to acquire more PK information. METHODS: Three PK studies were conducted in 12 healthy Chinese subjects (6 male, 6 female). Study 1 was a single-dose, three-period, three-dose level (10, 20, and 40 mg), three-sequence cross-over study under fasting conditions. Study 2 was a repeat-dose study (10 mg twice daily over 6 days; all 12 subjects). Study 3 was a two-period, two-sequence cross-over single-dose (10 mg) food interaction study. All randomizations (study 1, study 3) were done to ascertain 1:1 gender ratio per sequence. A validated liquid chromatography tandem mass spectrometry (LC/MS/MS) method was used to determine plasma Lafutidine concentrations. PK parameters were calculated by the non-compartmental method. RESULTS: The area under the time-concentration curve (AUC) and maximum plasma concentration (C max) of Lafutidine tablets were dose-independent in the single-dose study among these healthy volunteers. The PK parameters of the multiple-dose study were inconsistent with the single study. After administration of a single dose of 10 mg under either fed or fasting conditions, we found that food may not affect the degree of absorption of the Lafutidine tablets, but it may slow down the absorption rate. This is shown by the fact that the AUC showed no significant difference while the peak time was significantly delayed under fed conditions. CONCLUSION: The PK of Lafutidine showed dose proportionality. There was no significant accumulation of Lafutidine tablets with multiple dosing. Food did not affect the degree of Lafutidine absorption, but it did reduce the rate of absorption. Further study is needed regarding the effect of gender on Lafutidine. Lafutidine was well tolerated within the dose range 10-40 mg, and no serious adverse events were observed.
Interaction of lafutidine in binding to human serum albumin in gastric ulcer therapy: STD-NMR, WaterLOGSY-NMR, NMR relaxation times, Tr-NOESY, molecule docking, and spectroscopic studies.[Pubmed:27457418]
Arch Biochem Biophys. 2016 Sep 15;606:81-9.
In this study, Lafutidine (LAF) was used as a model compound to investigate the binding mechanism between antiulcer drugs and human serum albumin (HSA) through various techniques, including STD-NMR, WaterLOGSY-NMR, (1)H NMR relaxation times, tr-NOESY, molecule docking calculation, FT-IR spectroscopy, and CD spectroscopy. The analyses of STD-NMR, which derived relative STD (%) intensities, and WaterLOGSY-NMR, determined that LAF bound to HSA. In particular, the pyridyl group of LAF was in close contact with HSA binding pocket, whereas furyl group had a secondary binding. Competitive STD-NMR and WaterLOGSY-NMR experiments, with warifarin and ibuprofen as site-selective probes, indicated that LAF preferentially bound to site II in the hydrophobic subdomains IIIA of HSA. The bound conformation of LAF at the HSA binding site was further elucidated by transferred NOE effect (tr-NOESY) experiment. Relaxation experiments provided quantitative information about the relationship between the affinity and structure of LAF. The molecule docking simulations conducted with AutoDock and the restraints derived from STD results led to three-dimensional models that were consistent with the NMR spectroscopic data. The presence of hydrophobic forces and hydrogen interactions was also determined. Additionally, FT-IR and CD spectroscopies showed that LAF induced secondary structure changes of HSA.


