AF-DX 384Potent M2/M4 antagonist CAS# 118290-26-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118290-26-9 | SDF | Download SDF |
| PubChem ID | 119357 | Appearance | Powder |
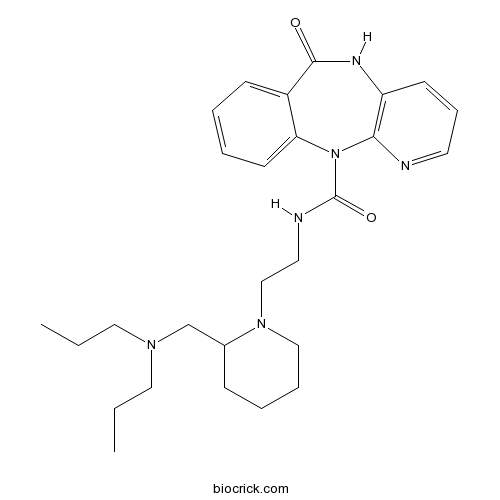
| Formula | C27H38N6O2 | M.Wt | 478.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | N-[2-[2-[(dipropylamino)methyl]piperidin-1-yl]ethyl]-6-oxo-5H-pyrido[2,3-b][1,4]benzodiazepine-11-carboxamide | ||
| SMILES | CCCN(CCC)CC1CCCCN1CCNC(=O)N2C3=CC=CC=C3C(=O)NC4=C2N=CC=C4 | ||
| Standard InChIKey | MZDYABXXPZNUCT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H38N6O2/c1-3-16-31(17-4-2)20-21-10-7-8-18-32(21)19-15-29-27(35)33-24-13-6-5-11-22(24)26(34)30-23-12-9-14-28-25(23)33/h5-6,9,11-14,21H,3-4,7-8,10,15-20H2,1-2H3,(H,29,35)(H,30,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent M2/M4 selective antagonist (pKi values are 8.22, 8.00, 7.51, 7.18 and 6.27 at human M2, M4, M1, M3 and M5 receptors respectively). |

AF-DX 384 Dilution Calculator

AF-DX 384 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0893 mL | 10.4463 mL | 20.8925 mL | 41.7851 mL | 52.2313 mL |
| 5 mM | 0.4179 mL | 2.0893 mL | 4.1785 mL | 8.357 mL | 10.4463 mL |
| 10 mM | 0.2089 mL | 1.0446 mL | 2.0893 mL | 4.1785 mL | 5.2231 mL |
| 50 mM | 0.0418 mL | 0.2089 mL | 0.4179 mL | 0.8357 mL | 1.0446 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.2089 mL | 0.4179 mL | 0.5223 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lafutidine
Catalog No.:BCC4544
CAS No.:118288-08-7
- Isodorsmanin A
Catalog No.:BCN6460
CAS No.:118266-99-2
- 1,4-Dicaffeoylquinic acid
Catalog No.:BCN5912
CAS No.:1182-34-9
- Soyasaponin Ab
Catalog No.:BCN2896
CAS No.:118194-13-1
- EMD638683
Catalog No.:BCC1551
CAS No.:1181770-72-8
- 2-Picenecarboxylic acid
Catalog No.:BCN3063
CAS No.:118172-80-8
- 6-Oxo-23-norpristimerol
Catalog No.:BCN8054
CAS No.:118172-79-5
- 6''-O-Acetylastragalin
Catalog No.:BCN6058
CAS No.:118169-27-0
- Volvaltrate B
Catalog No.:BCN6736
CAS No.:1181224-13-4
- [Phe8Ψ(CH-NH)-Arg9]-Bradykinin
Catalog No.:BCC5995
CAS No.:118122-39-7
- Schisanwilsonin I
Catalog No.:BCN5548
CAS No.:1181216-84-1
- Schisanwilsonin H
Catalog No.:BCN3315
CAS No.:1181216-83-0
- 6-O-Acetylscandoside
Catalog No.:BCN8320
CAS No.:118292-15-2
- Tazarotene
Catalog No.:BCC2540
CAS No.:118292-40-3
- Licoricesaponin A3
Catalog No.:BCN7905
CAS No.:118325-22-7
- Australine
Catalog No.:BCN2053
CAS No.:118396-02-4
- Trimethylamine oxide
Catalog No.:BCN1819
CAS No.:1184-78-7
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- MK 886
Catalog No.:BCC7017
CAS No.:118414-82-7
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- Arcyriaflavin A
Catalog No.:BCC7370
CAS No.:118458-54-1
- Cyclo(L-Phe-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3989
CAS No.:118477-06-8
The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia.[Pubmed:10353630]
Life Sci. 1999;64(19):1761-71.
Clinical studies of cholinergic pharmacotherapy, together with the putative role of the muscarinic receptor system in the neurophysiology of human behavior, support a possible muscarinic cholinergic involvement in schizophrenia. The present study has measured the density of [3H]AF-DX 384 labelled receptors (muscarinic M2 and M4) in the caudate-putamen, obtained at autopsy, from 19 subjects who had schizophrenia, and 20 subjects who did not have schizophrenia. [3H]AF-DX 384 binding was reduced in caudate-putamen from schizophrenic subjects (104 +/- 10.3 vs 145 +/- 901 fmol mg(-1) TE; mean +/- s.e.; p = 0.007). Preliminary analysis of patient drug data as well as rat studies suggest that the reduced [3H]AF-DX 384 binding in caudate-putamen of schizophrenic subjects is not wholly due to antipsychotic drug treatment, or anticholinergic medication for the treatment of extrapyramidal effects. These data suggest that the muscarinic cholinergic system may be involved in the pathology of schizophrenia.
Development of a new type of allosteric modulator of muscarinic receptors: hybrids of the antagonist AF-DX 384 and the hexamethonio derivative W84.[Pubmed:15163212]
J Med Chem. 2004 Jun 3;47(12):3324-7.
Various fragments of the hexamethonio-type allosteric agent W84 were linked to the secondary amino group of the muscarinic M(2) acetylcholine receptor-preferring antagonist AF-DX 384 to increase the area of attachment with the allosteric site. Addition of only the phthalimido moiety of W84 gave an allosteric enhancer of NMS binding. Thus, a new lead structure for the development of allosteric enhancers of NMS binding has been discovered.
Comparative distribution of binding of the muscarinic receptor ligands pirenzepine, AF-DX 384, (R,R)-I-QNB and (R,S)-I-QNB to human brain.[Pubmed:12297267]
J Chem Neuroanat. 2002 Sep;24(3):211-23.
Quinuclidinyl benzilate (QNB) and its derivatives are being developed to investigate muscarinic receptor changes in vivo in Alzheimer's disease and dementia with Lewy bodies. This is the first study of [125I]-(R,R)-I-QNB and [125I]-(R,S)-I-QNB binding in vitro in human brain. We have compared the in vitro binding of the muscarinic ligands [3H]pirenzepine and [3H]AF-DX 384, which have selectivity for the M1 and M2/M4 receptor subtypes, respectively, to the binding of [125I]-(R,R)-I-QNB and [125I]-(R,S)-I-QNB. This will provide a guide to the interpretation of in vivo SPET images generated with [123I]-(R,R)-I-QNB and [123I]-(R,S)-I-QNB. Binding was investigated in striatum, globus pallidus, thalamus and cerebellum, and cingulate, insula, temporal and occipital cortical areas, which show different proportions of muscarinic receptor subtypes, in post-mortem brain from normal individuals. M1 receptors are of high density in cortex and striatum and are relatively low in the thalamus and cerebellum, while M4 receptors are mainly expressed in the striatum, and M2 receptors are most evident in the cerebellum and thalamus. [125I]-(R,R)-I-QNB and [125I]-(R,S)-I-QNB density distribution patterns were consistent with binding to both M1 and M4 receptors, with [125I]-(R,R)-I-QNB additionally binding to a non-cholinergic site not displaceable by atropine. This distribution can be exploited by in vivo imaging, developing ligands for both SPET and PET, to reveal muscarinic receptor changes in Alzheimer's disease and dementia with Lewy bodies during the disease process and following cholinergic therapy.
Syntheses of R and S isomers of AF-DX 384, a selective antagonist of muscarinic M2 receptors.[Pubmed:10732976]
Bioorg Med Chem. 2000 Mar;8(3):591-600.
Enantiomers of 5,11-dihydro-11-[2-[2-[(N,N-dipropylaminomethyl)piperidin-1- yl]ethylamino]-carbonyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one (AF-DX 384) 1, have been synthesized from (S)-(+) and (R)-(-)-2-[N,N-dipropylaminomethyl]piperidine 4. The enantiomeric excess of 1 has been determined by capillary electrophoresis by using the alpha-highly sulphated cyclodextrin (alpha-HSCD) as chiral selector within the running electrolyte. (S)-(+)-(4) was prepared from (S)-(-)-pipecolic acid in a 4-step procedure (overall yield: 30%, ee: 99%) and (R)-(-)-AF-DX 384 from (R)-(+)-pipecolic acid. The (R)-(-) isomer exhibited in vitro a 23-fold higher affinity than its enantiomer (S)-(+) towards muscarinic receptors of subtype 2.
AF-DX 384 binding in rabbit cingulate cortex: two site kinetics and section autoradiography.[Pubmed:7616446]
J Pharmacol Exp Ther. 1995 Jul;274(1):562-9.
Autoradiographic studies of muscarinic receptors are limited by the lack of selective ligands. Inasmuch as AF-DX 384 has a higher affinity for m2 than m4 receptors and pirenzepine (PZ) has a reverse affinity profile, competition between these ligands was used to label m2 receptors in homogenized and sectioned tissue. Rabbit cingulate cortex was used because m2 receptors are expressed by anterior thalamic axons in posterior cingulate cortex (PCC) and this region is easily deafferented with undercut lesions to demonstrate presynaptic binding. Saturation isotherms and Scatchard analysis of [3H]AF-DX 384 binding showed one binding site with a KD of 9 +/- 2.3 nM (mean +/- SEM) and a Bmax of 1405 +/- 146 fmol/mg protein. Competition studies with [3H]AF-DX 384 (2 nM) and 10(-10)-10(-4) M PZ were performed in anterior cingulate cortex (ACC) and PCC. In both regions, the best fit was a two site model for low (BL) and high (BH) affinity binding in which Bmax values were similar (ACC: BL = 535 +/- 62 fmol/mg, BH = 676 +/- 85; PCC: BL = 552 +/- 41; BH = 675 +/- 85). Although affinities for KH were similar in each region (ACC: KH = 4.69 +/- 1.36 nM; PCC: KH = 8.53 +/- 3.69 nM), those for KL were significantly different (ACC: 181 +/- 15.4 nM; PCC: 285 +/- 42; P = .018). Binding of [3H]AF-DX 384 with PZ (150 nM) was best fit with a single site model (KD = 6 +/- 0.01 nM; Bmax = 688 +/- 31 fmol/mg), suggesting that PZ blocks the lower affinity site. (ABSTRACT TRUNCATED AT 250 WORDS)
Binding of [3H]AF-DX 384 to cloned and native muscarinic receptors.[Pubmed:1941609]
J Pharmacol Exp Ther. 1991 Nov;259(2):601-7.
The binding selectivity of [3H]AF-DX 384 [(+-)-5,11-dihydro-11- ([(2-(2-[(dipropylamino)methyl]-1- piperidinyl)ethyl)amino]carbonyl)-H-pyrido(2,3-b)(1,4)benzodiazepine-6-o ne] was evaluated with cloned human muscarinic receptors (M1-M4) in Chinese hamster ovary (CHO-K1) cell lines as well as in rat heart and brain. There were uniform classes of sites for the radioligand in the M2-rich tissues, heart (Kd = 2.3 nM) and brainstem (Kd = 2.4 nM). However, [3H]AF-DX 384 bound to all four cloned receptor subtypes. Using kinetic methods, the calculated Kd values were M2 (1 nm) greater than M4 (2.2 nM) greater than M3 (15 nM) greater than M1 (55 nM). Scatchard analysis with the CHO cells confirmed the high affinity of this radioligand for the M2 (1.8 nM) and M4 (2.5 nM) receptors. To evaluate the potential for selectively binding to M2 and M4 receptors in cortex and striatum, low concentrations (0.5-0.8 nM) of the radioligand were used and a two-site competition model was used to derive the binding constants for pirenzepine and AF-DX 116 [(+-)-11-2((-((diethylamino) methyl)-1-piperidinyl)acetyl)-5,11-dihydro-6H- pyrido(2,3-b)(1,4)-benzodiazepine-6-one] and to compare them with values obtained with cloned M2 and M4 receptors.(ABSTRACT TRUNCATED AT 250 WORDS)


