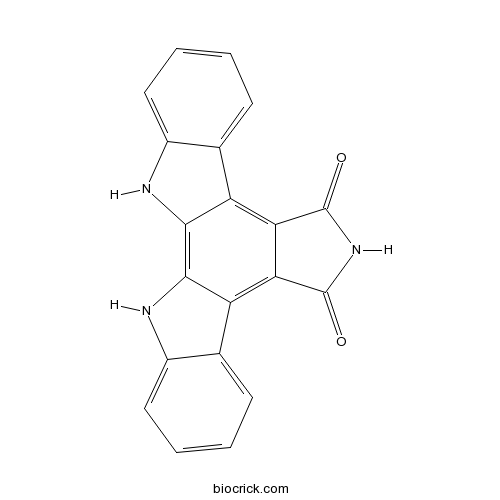
Arcyriaflavin Ainhibitor of human cytomegalovirus (HCMV) replication CAS# 118458-54-1 |
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118458-54-1 | SDF | Download SDF |
| PubChem ID | 5327723 | Appearance | Powder |
| Formula | C20H11N3O2 | M.Wt | 325.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| SMILES | C1=CC=C2C(=C1)C3=C4C(=C5C6=CC=CC=C6NC5=C3N2)C(=O)NC4=O | ||
| Standard InChIKey | KAJXOWFGKYKMMZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of cdk4/cyclin D1 (IC50 = 59 nM). Also active against CaM kinase II (IC50 = 25 nM) but displays selectivity over several other kinases in vitro (IC50 values for inhibition of PKA and PKC are > 2 and > 100 μM respectively). Inhibits human cytomegalovirus (HCMV) replication in vitro (IC50 = 200 nM). |

Arcyriaflavin A Dilution Calculator

Arcyriaflavin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0739 mL | 15.3695 mL | 30.739 mL | 61.4779 mL | 76.8474 mL |
| 5 mM | 0.6148 mL | 3.0739 mL | 6.1478 mL | 12.2956 mL | 15.3695 mL |
| 10 mM | 0.3074 mL | 1.5369 mL | 3.0739 mL | 6.1478 mL | 7.6847 mL |
| 50 mM | 0.0615 mL | 0.3074 mL | 0.6148 mL | 1.2296 mL | 1.5369 mL |
| 100 mM | 0.0307 mL | 0.1537 mL | 0.3074 mL | 0.6148 mL | 0.7685 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 0.2 μM for HCMV [1], 0.14 μM for D1–CDK4 [2]
The natural product Arcyriaflavin A, unsubstituted indolocarbazole, was a potent selective inhibitor of human cytomegalovirus (HCMV) replication. HCMV infection is typically unnoticed in healthy people, but can be life-threatening for the immunocompromised.
In vitro: Arcyriaflavin A is a potent, selective inhibitor of HCMV replication in cell culture, and the anti-HCMV activity appeared no relation to the inhibition of protein kinase C. The imide NH was identified to be essential for anti-HCMV activity [1]. Arcyriaflavin A also has been showed the inhibitory activity against D1/CDK4 with a IC50 of 59 nM. Based on X-ray co-crystal structure of staurosporine and the human CDK2, the acidic proton of the maleimide moiety and the carbonyl group play critical roles by acting as a hydrogen bond donor and acceptor in the ATP binding pocket of CDK2 [2].
In vivo: So far, no in vivo study has been conducted.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Slater MJ, Cockerill S, Baxter R, Bonser RW, Gohil K, Gowrie C, Robinson JE, Littler E, Parry N, Randall R, Snowden W. Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication. Bioorg Med Chem. 1999 Jun;7(6):1067-74.
[2] Zhu G, Conner S, Zhou X, Shih C, Brooks HB, Considine E, Dempsey JA, Ogg C, Patel B, Schultz RM, Spencer CD, Teicher B, Watkins SA. Synthesis of quinolinyl/isoquinolinyl[a]pyrrolo [3,4-c] carbazoles as cyclin D1/CDK4 inhibitors. Bioorg Med Chem Lett. 2003 Apr 7;13(7):1231-5.
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- MK 886
Catalog No.:BCC7017
CAS No.:118414-82-7
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- Trimethylamine oxide
Catalog No.:BCN1819
CAS No.:1184-78-7
- Australine
Catalog No.:BCN2053
CAS No.:118396-02-4
- Licoricesaponin A3
Catalog No.:BCN7905
CAS No.:118325-22-7
- Tazarotene
Catalog No.:BCC2540
CAS No.:118292-40-3
- 6-O-Acetylscandoside
Catalog No.:BCN8320
CAS No.:118292-15-2
- AF-DX 384
Catalog No.:BCC7024
CAS No.:118290-26-9
- Lafutidine
Catalog No.:BCC4544
CAS No.:118288-08-7
- Cyclo(L-Phe-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3989
CAS No.:118477-06-8
- Fmoc-D-Tyr(tBu)-OH
Catalog No.:BCC3569
CAS No.:118488-18-9
- 5,7-Dichlorokynurenic acid sodium salt
Catalog No.:BCC7758
CAS No.:1184986-70-6
- TRIS hydrochloride
Catalog No.:BCC7589
CAS No.:1185-53-1
- Myelin Basic Protein (87-99)
Catalog No.:BCC1028
CAS No.:118506-26-6
- LP 12 hydrochloride
Catalog No.:BCC7517
CAS No.:1185136-22-4
- DPPE fumarate
Catalog No.:BCC5669
CAS No.:1185241-83-1
- Sagittatoside A
Catalog No.:BCN2285
CAS No.:118525-35-2
- Sagittatoside B
Catalog No.:BCN2357
CAS No.:118525-36-3
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
- Icaritin
Catalog No.:BCN5352
CAS No.:118525-40-9
- Baohuoside V
Catalog No.:BCN2887
CAS No.:118544-18-6
Arcyriaflavin A monohydrate.[Pubmed:21285502]
Acta Crystallogr C. 2011 Feb;67(Pt 2):o57-9.
The asymmetric unit of the title compound comprises the monohydrated form of the natural product Arcyriaflavin A [systematic name: 12,13-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7(6H)-dione monohydrate], C(20)H(11)N(3)O(2).H(2)O. Individual molecular units are engaged in hydrogen-bonding interactions, forming two-dimensional zigzag supramolecular layers parallel to the (102) plane. The close packing of the layers is mediated by strong co-operative pi-pi stacking interactions, in tandem with interlayer hydrogen bonds involving the solvent water molecule.
Open analogues of arcyriaflavin A. Synthesis through Diels-Alder reaction between maleimides and 1-aryl-3-tert-butyldimethylsiloxy-1, 3-butadienes.[Pubmed:10843621]
J Org Chem. 2000 Jun 2;65(11):3387-94.
The preparation of a range of open analogues of Arcyriaflavin A is described. The synthetic approach is based on the use of perhydroisoindole-1,3,5-triones as key intermediates, which were obtained via Diels-Alder methodology using 1-aryl-3-siloxy-1, 3-butadienes as starting materials. Fischer indolization and aromatization processes afforded different methoxy-substituted arylpyrrolocarbazoles. The stereochemistry and conformation of the Diels-Alder products and the regiochemistry of the indolization reactions are supported by NMR and molecular modeling studies.
Homoarcyriaflavin: Synthesis of Ring-Expanded Arcyriaflavin Analogues.[Pubmed:11674727]
J Org Chem. 1999 Oct 29;64(22):8130-8137.
The construction of the ring-expanded carbazole system, forming arcyriaflavin homologues, is efficiently accomplished by the reaction of 2,2'-bridged bis-indoles with 3,4-dibromo-2,5-dihydro-1H-2,5-pyrroledione derivatives under Grignard conditions. A ring size of up to nine members in the central ring is achievable. Substitutions either at the indole system or at the imide-N are also possible. The conformation of homoarcyriaflavins as a cross-link between the rigid arcyriaflavins and the flexible arcyriarubins was investigated by NMR, X-ray, and semiempiric quantum chemical calculation methods.
Aryl[a]pyrrolo[3,4-c]carbazoles as selective cyclin D1-CDK4 inhibitors.[Pubmed:14552791]
Bioorg Med Chem Lett. 2003 Nov 3;13(21):3835-9.
The synthesis of new analogues of Arcyriaflavin A in which one indole ring is replaced by an aryl or heteroaryl ring is described. These new series of aryl[a]pyrrolo[3,4-c]carbazoles were evaluated as inhibitors of Cyclin D1-CDK4. A potent and selective D1-CDK4 inhibitor, 7a (D1-CDK4 IC(50)=45 nM), has been identified. The potency, selectivity profile against other kinases, and structure-activity relationship (SAR) trends of this class of compounds are discussed.
Synthesis of quinolinyl/isoquinolinyl[a]pyrrolo [3,4-c] carbazoles as cyclin D1/CDK4 inhibitors.[Pubmed:12657252]
Bioorg Med Chem Lett. 2003 Apr 7;13(7):1231-5.
A novel series of pyrrolo[3,4-c] carbazoles fused with a quinolinyl/isoquinolinyl moiety were synthesized and their D1/CDK4 inhibitory and antiproliferative activity were evaluated. Compound 8H, 14H-isoquinolinyl[6,5-a]-pyrrolo[3,4-c]carbazole-7,9-dione (1d) was found to be a highly potent D1/CDK4 inhibitor with an IC(50) of 69 nM. Compound 1d also inhibited tumor cell growth, arrested tumor cells in G1 phase and inhibited pRb phosphorylation.
Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication.[Pubmed:10428375]
Bioorg Med Chem. 1999 Jun;7(6):1067-74.
In our search for new, safer anti-HCMV agents, we discovered that the natural product Arcyriaflavin A (la) was a potent inhibitor of HCMV replication in cell culture. A series of analogues (symmetrical indolocarbazoles) was synthesised to investigate structure activity relationships in this series against a range of herpes viruses (HCMV, VZV, HSV1, and 2). This identified a number of novel, selective and potent inhibitors of HCMV, 12,13-dihydro-2,10-difluoro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol e-5,7-(6H)-dione (1d) being the best example (IC50=40 nM, therapeutic index > 1450). Compounds described in this series were generally poor inhibitors of protein kinase C betaII, and no correlation was found between the ability to inhibit HCMV and the enzyme PKC.


