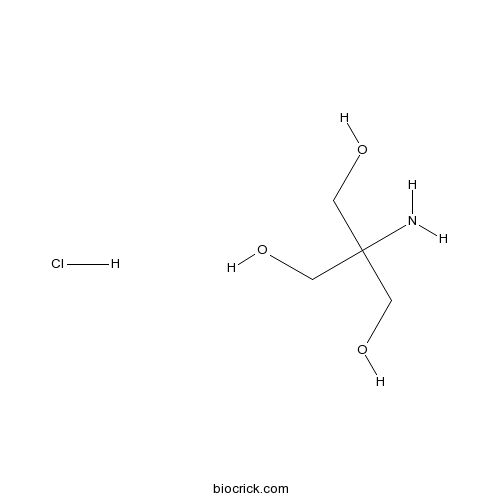
TRIS hydrochlorideCAS# 1185-53-1 |
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- QNZ (EVP4593)
Catalog No.:BCC2249
CAS No.:545380-34-5
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- JSH-23
Catalog No.:BCC4610
CAS No.:749886-87-1
- SC75741
Catalog No.:BCC5448
CAS No.:913822-46-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1185-53-1 | SDF | Download SDF |
| PubChem ID | 93573 | Appearance | Powder |
| Formula | C4H12ClNO3 | M.Wt | 157.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 4000 mM in water | ||
| Chemical Name | 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydrochloride | ||
| SMILES | C(C(CO)(CO)N)O.Cl | ||
| Standard InChIKey | QKNYBSVHEMOAJP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H11NO3.ClH/c5-4(1-6,2-7)3-8;/h6-8H,1-3,5H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Commonly used laboratory reagent |

TRIS hydrochloride Dilution Calculator

TRIS hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.3452 mL | 31.7259 mL | 63.4518 mL | 126.9036 mL | 158.6294 mL |
| 5 mM | 1.269 mL | 6.3452 mL | 12.6904 mL | 25.3807 mL | 31.7259 mL |
| 10 mM | 0.6345 mL | 3.1726 mL | 6.3452 mL | 12.6904 mL | 15.8629 mL |
| 50 mM | 0.1269 mL | 0.6345 mL | 1.269 mL | 2.5381 mL | 3.1726 mL |
| 100 mM | 0.0635 mL | 0.3173 mL | 0.6345 mL | 1.269 mL | 1.5863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7-Dichlorokynurenic acid sodium salt
Catalog No.:BCC7758
CAS No.:1184986-70-6
- Fmoc-D-Tyr(tBu)-OH
Catalog No.:BCC3569
CAS No.:118488-18-9
- Cyclo(L-Phe-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3989
CAS No.:118477-06-8
- Arcyriaflavin A
Catalog No.:BCC7370
CAS No.:118458-54-1
- Licoricesaponin G2
Catalog No.:BCN7897
CAS No.:118441-84-2
- MK 886
Catalog No.:BCC7017
CAS No.:118414-82-7
- nTZDpa
Catalog No.:BCC7268
CAS No.:118414-59-8
- UNC 926 hydrochloride
Catalog No.:BCC2445
CAS No.:1184136-10-4
- AG-18
Catalog No.:BCC1051
CAS No.:118409-57-7
- Trimethylamine oxide
Catalog No.:BCN1819
CAS No.:1184-78-7
- Australine
Catalog No.:BCN2053
CAS No.:118396-02-4
- Licoricesaponin A3
Catalog No.:BCN7905
CAS No.:118325-22-7
- Myelin Basic Protein (87-99)
Catalog No.:BCC1028
CAS No.:118506-26-6
- LP 12 hydrochloride
Catalog No.:BCC7517
CAS No.:1185136-22-4
- DPPE fumarate
Catalog No.:BCC5669
CAS No.:1185241-83-1
- Sagittatoside A
Catalog No.:BCN2285
CAS No.:118525-35-2
- Sagittatoside B
Catalog No.:BCN2357
CAS No.:118525-36-3
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
- Icaritin
Catalog No.:BCN5352
CAS No.:118525-40-9
- Baohuoside V
Catalog No.:BCN2887
CAS No.:118544-18-6
- H-Orn(2-Cl-Z)-OH
Catalog No.:BCC3002
CAS No.:118553-99-4
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
Semipreparative enantiomer separation of propranolol hydrochloride by high-performance liquid chromatography using cellulose tris(3,5-Dimethylphenylcarbamate) chiral stationary phase.[Pubmed:19007476]
J Chromatogr Sci. 2008 Oct;46(9):767-71.
In this paper, we describe the direct semipreparative resolution of racemic (rac)-propranolol hydrochloride by high-performance liquid chromatography using cellulose tris(3,5-dimethylphenylcarbamate) as chiral stationary phase and mobile phase systems containing petroleum ether and 2-propanol with the use of basic additives. At analytical scale, the retention factor of both enantiomers is less than 5 with the separation factor 1.95 and the resolution 2.4. Then, the analytical method is scaled up to semipreparative loading to obtain small amounts of both propranolol hydrochloride enantiomers. Petroleum ether, rather than n-hexane, is used to effectively reduce the production cost of (R)- and (S)-propranolol. To increase the throughput, overlapping injections are used, allowing an injection to be made every 6.5 min instead of every 12 min. At semipreparative scale, approximately 19 mg/h enantiomers are isolated. The first fraction [(R)-(+)-propranolol hydrochloride] is isolated with a purity of > 99.6% (e.e.) and > 97.0% yield, and the second [(S)-(-)-propranolol hydrochloride] is isolated with a purity of > 99.3% (e.e.) and > 95.0% yield. In addition, optical rotations of both propranolol hydrochloride enantiomers isolated are investigated.
Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution.[Pubmed:20190394]
Biol Pharm Bull. 2010;33(3):364-9.
Ascorbic acid (AA) has a strong anti-oxidant function evident as its ability to scavenge superoxide radicals in vitro. Moreover, AA is an essential ingredient for post-translational proline hydroxylation of collagen molecules. Dehydroascorbic acid (DHA), the oxidized form of AA, is generated from these reactions. In this study, we describe an improved method for assessing DHA in biological samples. The use of 35 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP) as a reductant completely reduced DHA to AA after 2 h on ice in a 5% solution of metaphosphoric acid containing 1 mM ethylenediaminetetraacetic acid (EDTA) at pH 1.5. This method enabled us to measure the DHA content in multiple tissues and plasma of 6-weeks-old mice. The percentages of DHA per total AA differed markedly among these tissues, i.e., from 0.8 to 19.5%. The lung, heart, spleen and plasma had the highest levels at more than 10% of DHA per total AA content, whereas the cerebrum, cerebellum, liver, kidney and small intestine had less than 5% of DHA per total AA content. This difference in DHA content may indicate an important disparity of oxidative stress levels among physiologic sites. Therefore, this improved method provides a useful standard for all DHA determinations.
High throughput method for the analysis of cetrizine hydrochloride in pharmaceutical formulations and in biological fluids using a tris(2,2'-bipyridyl)ruthenium(II)-peroxydisulphate chemiluminescence system in a two-chip device.[Pubmed:21726717]
Talanta. 2011 Aug 15;85(2):906-12.
A fast, economic and sensitive chemiluminescence (CL) method has been developed for the analysis of cetrizine hydrochloride (CET) in pharmaceutical formulations and in biological fluids. The CL method is based on the oxidation of tris(2,2'-bipyridyl)ruthenium(II) (Ru (bipy)(3)(2+)) by peroxydisulphate in a two-chip device. Up to 180 samples can be analysed per hour, consuming only minute quantities of reagents. Three instrumental setups were tested to find the most economical, sensitive and high throughput setup. In the first setup, a continuous flow of sample and CL reagents was used, whereas in the second setup, a fixed volume (2 muL) of (Ru (bipy)(3)(2+)) was introduced into a continuous infusion of peroxydisulphate and the sample. In the third design, a fixed volume of sample (2 muL) was injected while the CL reagents were continuously infused. Compared to the first setup, a 200% signal enhancement was observed in the third setup. Various parameters that influence the CL signal intensity, including pH, flow rates and reagent concentrations, were optimized. A linear response was observed over the range of 50 mug L(-1) to 6400 mug L(-1) (R(2)=0.9959) with RSD values of 1.1% (n=15) for 1000 mug L(-1). The detection limit was found to be 15 mug L(-1) (S/N=3). The amount of consumed sample was only 2 muL, from which the detected amount of CET was found to be 6.5 x 10(-14)mol. This procedure was successfully applied to the analysis of CET in pharmaceutical formulations and biological fluids.
Tris(azidoethyl)amine hydrochloride; a versatile reagent for synthesis of functionalized dumbbell oligodeoxynucleotides.[Pubmed:23339424]
Org Lett. 2013 Feb 1;15(3):694-7.
Triazole-cross-linked oligodeoxynucleotides were synthesized using the Cu(I) catalyzed alkyne-azide cycloaddition with tris(azidoethyl)amine hydrochloride and oligodeoxynucleotides possessing N-3-(propargyl)thymidine at both the 3'- and 5'-termini. Further installation of a functional molecule to the dumbbell oligodeoxynucleotides was achieved by utilizing the remaining azide group.


