Salvianolic acid CCAS# 115841-09-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115841-09-3 | SDF | Download SDF |
| PubChem ID | 13991590 | Appearance | Yellow powder |
| Formula | C26H20O10 | M.Wt | 492.44 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methanol and water | ||
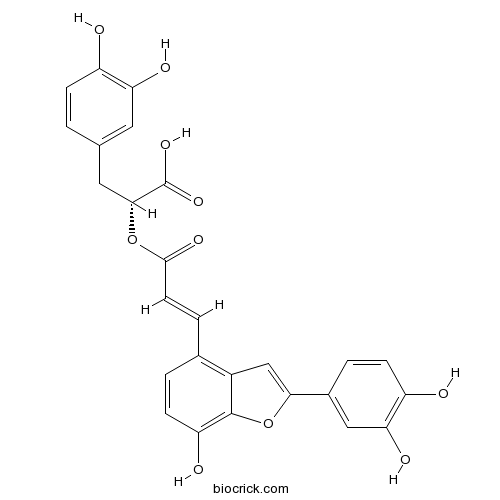
| Chemical Name | (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-[2-(3,4-dihydroxyphenyl)-7-hydroxy-1-benzofuran-4-yl]prop-2-enoyl]oxypropanoic acid | ||
| SMILES | C1=CC(=C(C=C1CC(C(=O)O)OC(=O)C=CC2=C3C=C(OC3=C(C=C2)O)C4=CC(=C(C=C4)O)O)O)O | ||
| Standard InChIKey | GCJWPRRNLSHTRY-VURDRKPISA-N | ||
| Standard InChI | InChI=1S/C26H20O10/c27-17-5-1-13(9-20(17)30)10-23(26(33)34)35-24(32)8-4-14-2-7-19(29)25-16(14)12-22(36-25)15-3-6-18(28)21(31)11-15/h1-9,11-12,23,27-31H,10H2,(H,33,34)/b8-4+/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Salvianolic acid C has antioxidant effect, it exhibits potent XOD inhibitory activity with an IC(50) of 9.07 μM. Salvianolic acid C enhances the inhibitory effects on sEH might be efficient ways to improve its cardiovascular protective and anti-inflammatory effects. |
| Targets | Immunology & Inflammation related | XOD |
| In vitro | In vitro inhibitory effects of ethanol extract of Danshen (Salvia miltiorrhiza) and its components on the catalytic activity of soluble epoxide hydrolase.[Pubmed: 25925966]Phytomedicine. 2015 Apr 15;22(4):444-51.Soluble epoxide hydrolase (sEH) has been demonstrated to be a key enzyme involved in the pathologic development of several cardiovascular diseases and inflammation, and inhibition of sEH is therefore very helpful or crucial for the treatment of ischemia-reperfusion injury, cardiac hypertrophy, hypertension and inflammation. Danshen, the dried root of Salvia miltiorrhiza (Fam. Labiatae), has been used for the treatment of cardiovascular and cerebrovascular diseases in China and other countries for hundreds of years. Recent studies indicated that Danshen and its preparations also have potential for the management of inflammation. However, little information is available about the possibility of Danshen and its components on sEH inhibition.
|

Salvianolic acid C Dilution Calculator

Salvianolic acid C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0307 mL | 10.1535 mL | 20.307 mL | 40.6141 mL | 50.7676 mL |
| 5 mM | 0.4061 mL | 2.0307 mL | 4.0614 mL | 8.1228 mL | 10.1535 mL |
| 10 mM | 0.2031 mL | 1.0154 mL | 2.0307 mL | 4.0614 mL | 5.0768 mL |
| 50 mM | 0.0406 mL | 0.2031 mL | 0.4061 mL | 0.8123 mL | 1.0154 mL |
| 100 mM | 0.0203 mL | 0.1015 mL | 0.2031 mL | 0.4061 mL | 0.5077 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,7-Dideacetoxytaxinine J
Catalog No.:BCN7281
CAS No.:115810-14-5
- Tubeimoside III
Catalog No.:BCN2956
CAS No.:115810-13-4
- Tubeimoside II
Catalog No.:BCN2955
CAS No.:115810-12-3
- ent-Atisane-3beta,16alpha,17-triol
Catalog No.:BCN6626
CAS No.:115783-44-3
- 15,16-Dihydro-15-methoxy-16-oxohardwickiic acid
Catalog No.:BCN1612
CAS No.:115783-35-2
- Galanolactone
Catalog No.:BCN6037
CAS No.:115753-79-2
- 1-O-galloyl-6-O-cinnamoylglucose
Catalog No.:BCN8264
CAS No.:115746-69-5
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- B2
Catalog No.:BCC7505
CAS No.:115687-05-3
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- Aurora A Inhibitor I
Catalog No.:BCC2182
CAS No.:1158838-45-9
- N1-Methoxymethyl picrinine
Catalog No.:BCN6038
CAS No.:1158845-78-3
- Tos-Arg-OH
Catalog No.:BCC2873
CAS No.:1159-15-5
- Angoroside C
Catalog No.:BCN4997
CAS No.:115909-22-3
- Tarafenacin D-tartrate
Catalog No.:BCC4148
CAS No.:1159101-48-0
- Salvianolic acid B
Catalog No.:BCN6106
CAS No.:115939-25-8
- Alstonic acid A
Catalog No.:BCN6039
CAS No.:1159579-44-8
- Alstonic acid B
Catalog No.:BCN6040
CAS No.:1159579-45-9
- Poricoic acid AE
Catalog No.:BCN7282
CAS No.:1159753-88-4
- CZC24832
Catalog No.:BCC1507
CAS No.:1159824-67-5
- Abiesadine N
Catalog No.:BCN6041
CAS No.:1159913-80-0
- Caulophine
Catalog No.:BCN7990
CAS No.:1159989-19-1
In vitro inhibitory effects of ethanol extract of Danshen (Salvia miltiorrhiza) and its components on the catalytic activity of soluble epoxide hydrolase.[Pubmed:25925966]
Phytomedicine. 2015 Apr 15;22(4):444-51.
BACKGROUND: Soluble epoxide hydrolase (sEH) has been demonstrated to be a key enzyme involved in the pathologic development of several cardiovascular diseases and inflammation, and inhibition of sEH is therefore very helpful or crucial for the treatment of ischemia-reperfusion injury, cardiac hypertrophy, hypertension and inflammation. Danshen, the dried root of Salvia miltiorrhiza (Fam. Labiatae), has been used for the treatment of cardiovascular and cerebrovascular diseases in China and other countries for hundreds of years. Recent studies indicated that Danshen and its preparations also have potential for the management of inflammation. However, little information is available about the possibility of Danshen and its components on sEH inhibition. PURPOSE AND METHODS: Danshen extracts and its constituents were tested for sEH inhibition using its physiological substrate, 8,9-EET, based on a LC-MS/MS assay in this study. RESULTS: Among the tested 15 compounds, tanshinone IIA and cryptotanshinone were found to be the potent (Ki = 0.87 muM) and medium (Ki = 6.7 muM) mixed-type inhibitors of sEH, respectively. Salvianolic acid C (Ki = 8.6 muM) was proved to be a moderate noncompetitive sEH inhibitor. In consistent with the inhibition results of the pure compounds, the 75% ethanol extract of Danshen (EE, IC50 = 86.5 mug/ml) which contained more tanshinone IIA and cryptotanshinone exhibited more potent inhibition on sEH than the water extract (WE, IC50 > 200 mug/ml) or 1 M NaHCO3 (BE, IC50 > 200 mug/ml) extract. CONCLUSION: These data indicated that using the ethanol fraction of Danshen and increasing the amounts of tanshinone IIA, cryptotanshinone and Salvianolic acid C, especially the contents of tanshinone IIA in Danshen extract or preparations to enhance the inhibitory effects on sEH might be efficient ways to improve its cardiovascular protective and anti-inflammatory effects, and that herbal medicines could be an untapped reservoir for sEH-inhibition agents and developing sEH inhibitors from the cardiovascular protective and anti-inflammatory herbs is a promising approach.


