GalanolactoneCAS# 115753-79-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115753-79-2 | SDF | Download SDF |
| PubChem ID | 6450044 | Appearance | Powder |
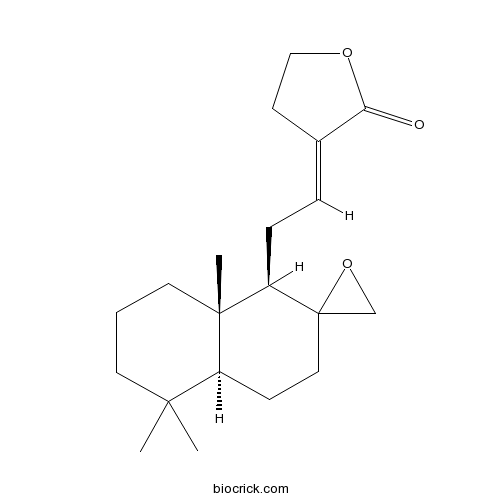
| Formula | C20H30O3 | M.Wt | 318.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3E)-3-[2-[(1R,4aS,8aS)-5,5,8a-trimethylspiro[3,4,4a,6,7,8-hexahydro-1H-naphthalene-2,2'-oxirane]-1-yl]ethylidene]oxolan-2-one | ||
| SMILES | CC1(CCCC2(C1CCC3(C2CC=C4CCOC4=O)CO3)C)C | ||
| Standard InChIKey | MBPTXJNHCBXMBP-BQORUNDISA-N | ||
| Standard InChI | InChI=1S/C20H30O3/c1-18(2)9-4-10-19(3)15(18)7-11-20(13-23-20)16(19)6-5-14-8-12-22-17(14)21/h5,15-16H,4,6-13H2,1-3H3/b14-5+/t15-,16+,19-,20?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Galanolactone shows cytotoxic activity . 2. Galanolactone has anti-5-HT effect, a diterpenoid isolated from ginger, is related to antagonism of 5-HT3 receptors. 3. Galanolactone exerts anti-obesity effect through down regulation of adipogenic transcription factors and adipogenic marker genes. 4. Galanolactone has inhibit nitric oxide production with IC(50) values ranging from 5.5 to 28.5 μM. |
| Targets | NO | 5-HT Receptor |

Galanolactone Dilution Calculator

Galanolactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1397 mL | 15.6986 mL | 31.3972 mL | 62.7943 mL | 78.4929 mL |
| 5 mM | 0.6279 mL | 3.1397 mL | 6.2794 mL | 12.5589 mL | 15.6986 mL |
| 10 mM | 0.314 mL | 1.5699 mL | 3.1397 mL | 6.2794 mL | 7.8493 mL |
| 50 mM | 0.0628 mL | 0.314 mL | 0.6279 mL | 1.2559 mL | 1.5699 mL |
| 100 mM | 0.0314 mL | 0.157 mL | 0.314 mL | 0.6279 mL | 0.7849 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-O-galloyl-6-O-cinnamoylglucose
Catalog No.:BCN8264
CAS No.:115746-69-5
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- B2
Catalog No.:BCC7505
CAS No.:115687-05-3
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- Cinnamyl caffeate
Catalog No.:BCN7721
CAS No.:115610-32-7
- Cinnamyl isoferulate
Catalog No.:BCN7718
CAS No.:115610-31-6
- Cinnamyl coumarate
Catalog No.:BCN7739
CAS No.:115610-30-5
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- 15,16-Dihydro-15-methoxy-16-oxohardwickiic acid
Catalog No.:BCN1612
CAS No.:115783-35-2
- ent-Atisane-3beta,16alpha,17-triol
Catalog No.:BCN6626
CAS No.:115783-44-3
- Tubeimoside II
Catalog No.:BCN2955
CAS No.:115810-12-3
- Tubeimoside III
Catalog No.:BCN2956
CAS No.:115810-13-4
- 2,7-Dideacetoxytaxinine J
Catalog No.:BCN7281
CAS No.:115810-14-5
- Salvianolic acid C
Catalog No.:BCN5376
CAS No.:115841-09-3
- Aurora A Inhibitor I
Catalog No.:BCC2182
CAS No.:1158838-45-9
- N1-Methoxymethyl picrinine
Catalog No.:BCN6038
CAS No.:1158845-78-3
- Tos-Arg-OH
Catalog No.:BCC2873
CAS No.:1159-15-5
- Angoroside C
Catalog No.:BCN4997
CAS No.:115909-22-3
- Tarafenacin D-tartrate
Catalog No.:BCC4148
CAS No.:1159101-48-0
- Salvianolic acid B
Catalog No.:BCN6106
CAS No.:115939-25-8
Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger.[Pubmed:2054863]
Chem Pharm Bull (Tokyo). 1991 Feb;39(2):397-9.
It has been reported that an acetone extract of ginger and its fractions have anti-5-HT (5-hydroxytryptamine; serotonin) effects. In the present study, guinea pig ileum, rat stomach fundus and rabbit aortic strips are used in order to determine the constituents of fraction 2 which are responsible for anti-5-HT effect and to examine their pharmacological properties. The analysis of fraction 2-3 indicated that Galanolactone, a diterpenoid, is one of the active constituents. In guinea pig ileum, Galanolactone inhibited contractile responses to 5-HT with a pIC50 value 4.93. pIC50 value of Galanolactone against the response to 2-methyl-5-HT, a selective 5-HT3 agonist, in the presence of methysergide at 1 x 10(-5) M was 5.10. pIC50 values of ICS 205-930, a selective 5-HT3 antagonist, were 5.30 and 7.49, respectively. The concentration-response curve of 5-HT was shown as a biphasic curve and Galanolactone caused a selective shift to the right of the second phase. In the same preparations, the pIC50 value of Galanolactone and ICS 205-930 against the response to carbamylcholine (CCh) was 4.45 and 4.46. The inhibitory effect of Galanolactone on the 5-HT response in the stomach fundus and aortic strips was less than that in the ileum. In addition, in the thoracic aorta precontracted with 50 mM K+, the relaxing effect of Galanolactone was about 1/10 of that of papaverine. These results suggest that the anti-5-HT effect of Galanolactone, a diterpenoid isolated from ginger, is related to antagonism of 5-HT3 receptors.
Phenylpropanoid ester from Zingiber officinale and their inhibitory effects on the production of nitric oxide.[Pubmed:22370785]
Arch Pharm Res. 2012 Feb;35(2):315-20.
A new phenylpropanoid ester mixture, (E)-geranylferulic acid (1a) and (Z)-geranylferulic acid (1b), along with 13 known compounds, [6]-gingerol (2), [8]-gingerol (3), [10]-gingerdione (4), 1-dehydro-[6]-gingerdione (5), 1-dehydro-[8]-gingerdione (6), [6]-paradol (7), [8]-paradol (8), [6]-gingeroldiacetate (9), 6-hydroxy-[6]-shogaol (10), Galanolactone (11), trans-(R)-sesquiphellandrol (12), trans-sesquipiperitol (13), and 4alpha,5beta-dihydroxybisabola-2,10-diene (14) were isolated from ethanol extract of Zingiber officinale. Their structures were determined based on the spectroscopic (1D, 2D-NMR and MS) and chemical evidence. All of the isolates were evaluated for their potential to inhibit LPS-induced production of nitric oxide in murine macrophage RAW264.7 cells. Compounds 1-12 were found to inhibit nitric oxide production with IC(50) values ranging from 5.5 to 28.5 muM.
Antiplasmodial activity of labdanes from Aframomum latifolium and Aframomum sceptrum.[Pubmed:12143001]
Planta Med. 2002 Jul;68(7):642-4.
Bioguided fractionation of extracts of Aframomum latifolium and A. sceptrum (Zingiberaceae) resulted in isolation of (+)-( S)-nerolidol and seven labdanes, coronarin B, galanal A and B, Galanolactone, ( E)-8beta,17-epoxylabd-12-ene-15,16-dial, (+)-( E)-labda-8(17), 12-diene-15,16-dial and its diethyl acetal, the latter being presumably an isolation artefact. The labdanes show a modest in vitro activity against a chloroquine-sensitive Plasmodium falciparum strain.


