Tofacitinib (CP-690550,Tasocitinib)Janus kinase inhibitor CAS# 477600-75-2 |
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- GLPG0634
Catalog No.:BCC4145
CAS No.:1206161-97-8
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

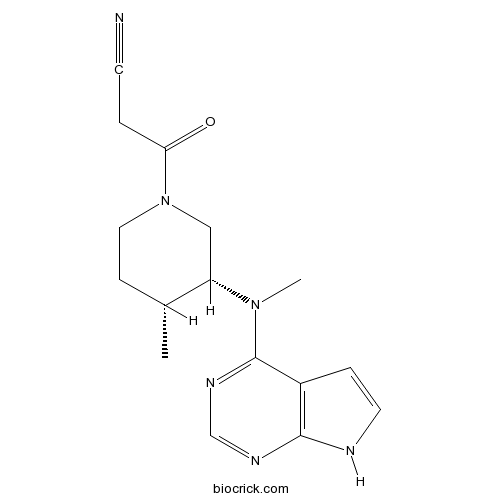
| Cas No. | 477600-75-2 | SDF | Download SDF |
| PubChem ID | 9926791 | Appearance | Powder |
| Formula | C16H20N6O | M.Wt | 312.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tasocitinib; CP-690550 | ||
| Solubility | DMSO : 65 mg/mL (208.09 mM; Need ultrasonic) H2O : 0.15 mg/mL (0.48 mM; Need ultrasonic and warming) | ||
| Chemical Name | 3-[(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile | ||
| SMILES | CC1CCN(CC1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N | ||
| Standard InChIKey | UJLAWZDWDVHWOW-YPMHNXCESA-N | ||
| Standard InChI | InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tofacitinib inhibits JAK3 with IC50 of 1 nM while inhibiting JAK2, JAK1, Rock-II and Lck with IC50 values of 20, 112, 3400 and 3870 nM, respectively.In Vitro:Tofacitinib (CP-690550) citrate binds potentially at JAK3 and JAK2 as 2.2 nM and 5 nM (Kd). The report includes additional binding for Tofacitinib at Camk1 (Kd of 5,000 nM), DCamkL3 (Kd of 4.5 nM), Mst2 (Kd of 4,300 nM), Pkn1 (Kd of 200 nM), Rps6ka2 (Kin.Dom.2-C-terminal) (Kd of 1,400 nM), Rps6ka6 (Kin.Dom.2-C-terminal) (Kd of 1,200 nM), Snark (Kd of 420 nM), Tnk1 (Kd of 640 nM) and Tyk2 (Kd of 620 nM)[1].In Vivo:Animals that are treated with Tofacitinib show a significantly lower production of anti-drug antibodies (ADAs) compare with PEG-treated control mice (for five weeks after initial immunization, p<0.01, n=8). Moreover ADAs become detectable earliest on day 28. A difference of 1000- to 200-fold in titers to SS1P is apparent from days 21 through 35, respectively. Compare to SS1P, mice injected with keyhole limpet hemocyanin (KLH) generate a more rapid antibody response. Yet, the administration of Tofacitinib reduces anti-KLH titers compare to controls (p<0.05 on day 21, p<0.01 on day 28, respectively, n=5). Reductions in titers ranged from 5000- to 250-fold from days 21 through 28, respectively[2]. Based on previous dose-response studies, a daily dose of Tofacitinib of 6.2 mg/kg is selected to provide 80% inhibition of hind paw volume and plasma exposure capable of suppressing the JAK1 and JAK3 signaling pathways for >4 hours[3]. References: | |||||

Tofacitinib (CP-690550,Tasocitinib) Dilution Calculator

Tofacitinib (CP-690550,Tasocitinib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2013 mL | 16.0067 mL | 32.0133 mL | 64.0266 mL | 80.0333 mL |
| 5 mM | 0.6403 mL | 3.2013 mL | 6.4027 mL | 12.8053 mL | 16.0067 mL |
| 10 mM | 0.3201 mL | 1.6007 mL | 3.2013 mL | 6.4027 mL | 8.0033 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6403 mL | 1.2805 mL | 1.6007 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6403 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tofacitinib, also named CP-690550 orTasocitinib, is a novel oral Janus kinase inhibitor which is being used as a targeted immune-modulator. It primarily inhibits signaling through heterodimeric receptors associated with JAK3, JAK1, or both of them, with functional selectivity over JAK2-paired receptors. Inhibition of JAK1 and JAK3 by tofacitinib blocks signaling for several cytokines, including interleukins 2, 4, 7, 9, 15, and 21. These cytokines are integral to lymphocyte activation, function, and proliferation. Tofacitinib is also an inhibitor of STAT molecules. Recent efforts to investigate the mechanism of action have shown that tofacitinib interacts with multiple JAKs and presumably other kinases
References
Roy Fleischmann, Joel Kremer, John Cush, Hendrik Schulze-Koops, Carol A. Connell, John D. Bradley, David Gruben, Gene V. Wallenstein, Samuel H. Zwillich, and Keith S. Kanik. Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. N Engl J Med 2012; 367:495-507
William J. Sandborn, Subrata Ghosh, Julian Panes, Ivana Vranic, Chinyu Su, Samantha Rousell, Wojciech Niezychowski. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. N Engl J Med 2012; 367:616-624.
Keisuke Maeshima, Kunihiro Yamaoka, Satoshi Kubo, Kazuhisa Nakano, Shigeru Iwata, Kazuyoshi Saito, Masanobu Ohishi, Hisaaki Miyahara, Shinya Tanaka, Koji Ishii, Hironobu Yoshimatsu, Yoshiya Tanaka. The JAK Inhibitor Tofacitinib Regulates Synovitis Through Inhibition of Interferon- and Interleukin-17 Production by Human CD4 T Cells. Arthritis & Rheumatism. 2012. 64(6): 1790–1798.
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- Musellarin A
Catalog No.:BCN7186
CAS No.:477565-36-9
- Sculponeatin K
Catalog No.:BCN5537
CAS No.:477529-70-7
- Ophiopogonanone C
Catalog No.:BCN6620
CAS No.:477336-75-7
- cis-ACPD
Catalog No.:BCC6566
CAS No.:477331-06-9
- (±)-AC 7954 hydrochloride
Catalog No.:BCC7381
CAS No.:477313-09-0
- Mangiferin
Catalog No.:BCN5535
CAS No.:4773-96-0
- Bergenin
Catalog No.:BCN5540
CAS No.:477-90-7
- Digitolutein
Catalog No.:BCN3089
CAS No.:477-86-1
- Obtusifolin
Catalog No.:BCN2537
CAS No.:477-85-0
- Damnacanthal
Catalog No.:BCN5539
CAS No.:477-84-9
- Isochondodendrine
Catalog No.:BCC9234
CAS No.:477-62-3
- PIM-1 Inhibitor 2
Catalog No.:BCC2446
CAS No.:477845-12-8
- 3-(2-Glucosyloxy-4-methoxyphenyl)propanoic acid
Catalog No.:BCN7068
CAS No.:477873-63-5
- Neostenine
Catalog No.:BCN5541
CAS No.:477953-07-4
- Nobiletin
Catalog No.:BCN5542
CAS No.:478-01-3
- Lucidin
Catalog No.:BCC1709
CAS No.:478-08-0
- Pseudoaspidin
Catalog No.:BCN6386
CAS No.:478-28-4
- Eleutherin
Catalog No.:BCN8475
CAS No.:478-36-4
- Droserone
Catalog No.:BCN7985
CAS No.:478-40-0
- Rhein
Catalog No.:BCN5947
CAS No.:478-43-3
- Berbamine
Catalog No.:BCN5543
CAS No.:478-61-5
- Pseudococaine
Catalog No.:BCN1902
CAS No.:478-73-9
- PHA 543613 hydrochloride
Catalog No.:BCC5972
CAS No.:478149-53-0
Effectiveness of biologic and non-biologic antirheumatic drugs on anaemia markers in 153,788 patients with rheumatoid arthritis: New evidence from real-world data.[Pubmed:28947313]
Semin Arthritis Rheum. 2018 Feb;47(4):478-484.
BACKGROUND: To evaluate the impact of treatment with disease-modifying antirheumatic drugs (DMARDs), including IL-6 receptor inhibitor tocilizumab (TCZ), on anaemia markers in patients with rheumatoid arthritis. METHODS: Using the Centricity Electronic Medical Records from USA, patients with rheumatoid arthritis diagnosed between January 2000 and April 2016, who initiated TCZ (n = 3732); tofacitinib (TOFA, n = 3126); other biologic DMARD (obDMARD, n = 55,964); or other non-biologic DMARD (onbDMARD, n = 91,236) were identified. Changes in haemoglobin (Hb) and haematocrit (Hct) over 2 years of treatment initiation were evaluated, adjusting and balancing for confounders. RESULTS: Mean (95% CI) adjusted increase in Hb and Hct levels at 24 months in TCZ group were 0.23g/dL (0.14, 0.42) and 0.96% (0.41, 1.52) respectively. Among patients with anaemia in the TCZ group, Hb and Hct increased significantly by 0.72g/dL and 2.06%, respectively. Patients in the TCZ group were 86% (95% CI of OR: 1.43, 2.00) more likely to increase Hb >/= 1g/dL compared to the other groups combined. No clinically significant changes in Hb were observed in the other groups. The obDMARD group demonstrated lower Hct increase than TCZ group, while no significant changes were observed in the remaining groups. Compared to those who initiated TCZ therapy after 1 year of diagnosis of rheumatoid arthritis, those who initiated earlier were 95% (OR = 1.95; 95% CI: 1.19, 3.21; p < 0.001) more likely to increase Hb within 6 months. CONCLUSIONS: This real-world study suggests significant increase in Hb and Hct levels after TCZ therapy in anaemic and non-anaemic patients with rheumatoid arthritis, compared with other biologic and non-biologic DMARDs.
Effects of tofacitinib in early arthritis-induced bone loss in an adjuvant-induced arthritis rat model.[Pubmed:28968875]
Rheumatology (Oxford). 2018 Aug 1;57(8):1461-1471.
Objectives: The main goal of this work was to analyse how treatment intervention with tofacitinib prevents the early disturbances of bone structure and mechanics in the rat model of adjuvant-induced arthritis. This is the first study to access the impact of tofacitinib on the skeletal bone effects of inflammation. Methods: Fifty Wistar rats with adjuvant-induced arthritis were randomly housed in experimental groups, as follows: non-arthritic healthy group (n = 20); arthritic non-treated group (n = 20); and 10 animals undergoing tofacitinib treatment. Rats were monitored during 22 days after disease induction for the inflammatory score, ankle perimeter and body weight. Healthy non-arthritic rats were used as controls for comparison. After 22 days of disease progression, rats were killed and bone samples collected for histology, micro-CT, three-point bending and nanoindentation analysis. Blood samples were also collected for quantification of bone turnover markers and systemic cytokines. Results: At the tissue level, measured by nanoindentation, tofacitinib increased bone cortical and trabecular hardness. However, micro-CT and three-point bending tests revealed that tofacitinib did not reverse the effects of arthritis on the cortical and trabecular bone structure and on mechanical properties. Conclusion: Possible reasons for these observations might be related to the mechanism of action of tofacitinib, which leads to direct interactions with bone metabolism, and/or to the kinetics of its bone effects, which might need longer exposure.
Design and Synthesis of a Highly Selective JAK3 Inhibitor for the Treatment of Rheumatoid Arthritis.[Pubmed:28944566]
Arch Pharm (Weinheim). 2017 Nov;350(11).
Selective inhibition of Janus kinase 3 (JAK3) has been identified as an important strategy for the treatment of autoimmune disorders. Based on the unique cysteine 909 residue (Cys909) of JAK3 at the gatekeeper position, we have developed a new irreversible covalent inhibitor, III-4, which is highly potent and selective in targeting JAK3. Importantly, III-4 selectively inhibited JAK3 (IC50 = 57 +/- 1.21 nM) over other JAKs (IC50 > 10 microM) and Cys909 kinome members (IC50 > 1 microM). A cellular selectivity study also confirmed that III-4 preferentially inhibited JAK3 over JAK1 in JAK/STAT signaling. Moreover, the fact that III-4 covalently modified the Cys909 residue in JAK3 was clearly validated by mass spectrometry and covalent docking analysis. Based on the favorable target profiles, the pharmacokinetic properties and its low toxicity, III-4 exhibited better efficacy than tofacitinib in impeding disease progression in CIA mice, without any significant adverse effects. Taken together, III-4 is a potent, selective, and durable inhibitor of JAK3 and has the potential for the treatment of inflammatory disorders and autoimmune diseases, such as rheumatoid arthritis.
Tofacitinib in psoriatic arthritis.[Pubmed:28967798]
Immunotherapy. 2017 Nov;9(14):1153-1163.
Psoriatic arthritis is a heterogeneous disease that has been difficult to manage until the recent advent of biologics. However, there are still unmet medical needs for newer agents. Tofacitinib is a Janus family of kinases inhibitor approved for treating rheumatoid arthritis in many countries and psoriasis in Russia. We reviewed the evidences of tofacitinib in psoriatic arthritis treatment. The efficacy and safety profiles result from Phase III clinical trials (OPAL BROADEN and OPAL BEYOND) and one open-label extension study (OPAL BALANCE). Both tofacitinib 5 or 10 mg twice a day were superior to placebo for American College of Rheumatology 20% improvement criteria response at 3 months and showed significant improvement of skin, enthesitis and dactylitis. Tofacitinib is a promising treatment option for psoriatic arthritis.
Newer treatments of psoriasis regarding IL-23 inhibitors, phosphodiesterase 4 inhibitors, and Janus kinase inhibitors.[Pubmed:28994166]
Dermatol Ther. 2017 Nov;30(6).
The rapid progress of genetic engineering furthermore opens up new prospects in the therapy of this difficult-to-treat disease. IL-23 inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and Janus kinase (JAK) inhibitors are currently encouraging further research. Two drugs which are IL-23 inhibitors are now in phase III of clinical trials. The aim of the action of both drugs is selective IL-23 inhibition by targeting the p19 subunit. Guselkumab is a fully human monoclonal antibody. Tildrakizumab is a humanized monoclonal antibody, which also belongs to IgG class and is targeted to subunit p19 of interleukin 23 (IL-23). Phosphodiesterase inhibitors exert an anti-inflammatory action and their most common group is the PDE4 family. PDE4 inhibits cAMP, which reduces the inflammatory response of the pathway of Th helper lymphocytes, Th17, and type 1 interferon which modulates the production of anti-inflammatory cytokines such as IL-10 interleukins. The Janus kinase (JAK) signaling pathway plays an important role in the immunopathogenesis of psoriasis. Tofacitinib suppresses the expression of IL-23, IL-17A, IL-17F, and IL-22 receptors during the stimulation of lymphocytes. Ruxolitinib is a selective inhibitor of JAK1 and JAK2 kinases and the JAK-STAT signaling pathway. This article is a review of the aforementioned drugs as described in the latest available literature.


