Bufo gargarizans
Bufo gargarizans
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Bufo gargarizans
- Cat.No. Product Name CAS Number COA
-
BCN2283
Cinobufotalin1108-68-5
Instructions

-
BCN2297
Bufarenogin17008-65-0
Instructions
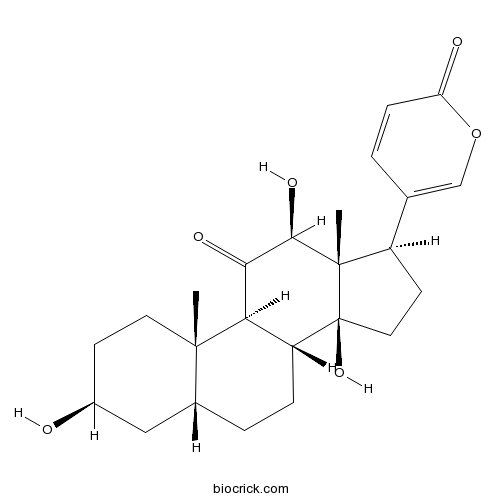
-
BCN8234
Pseudobufarenogin17008-69-4
Instructions
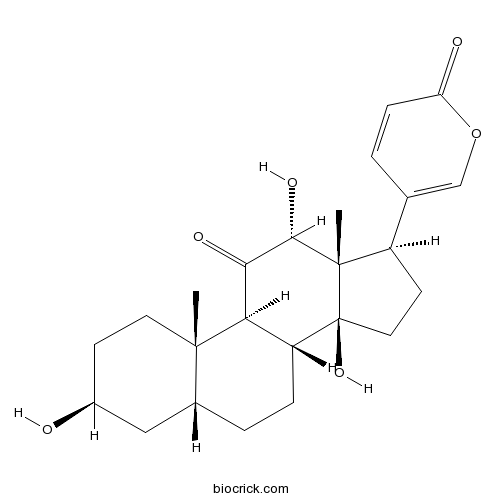
-
BCN8230
Resibufagin20987-24-0
Instructions
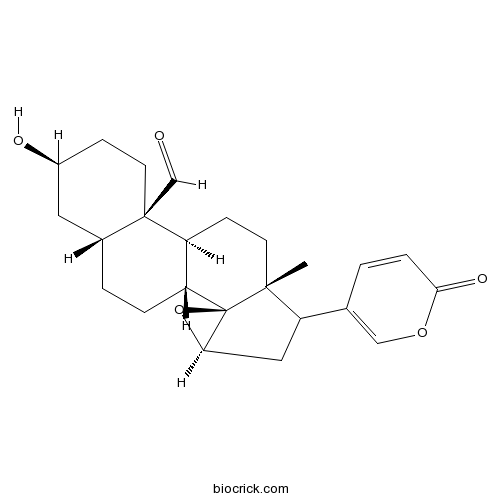
-
BCN8229
19-Oxocinobufagin24512-59-2
Instructions

-
BCN8233
19-Oxocinobufotalin24512-60-5
Instructions

-
BCN7837
Arenobufagin 3-hemisuberate30219-16-0
Instructions
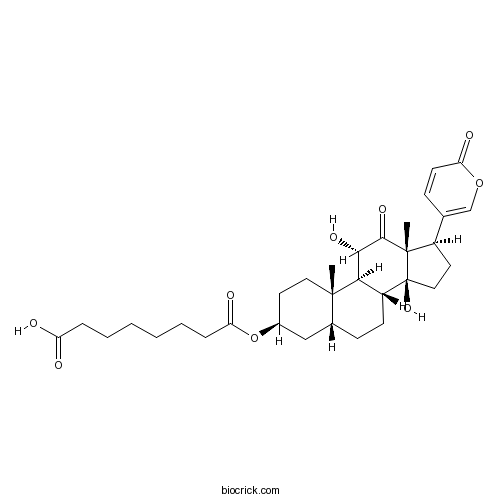
-
BCN8237
19-Hydroxybufalin39844-86-5
Instructions

-
BCN2720
Deacetylcinobufagin4026-95-3
Instructions
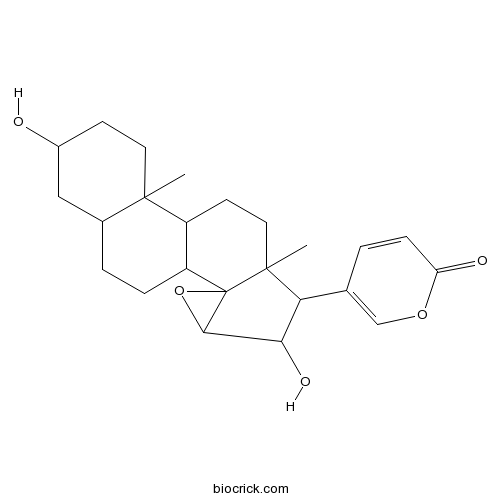
-
BCN5401
Arenobufagin464-74-4
Instructions
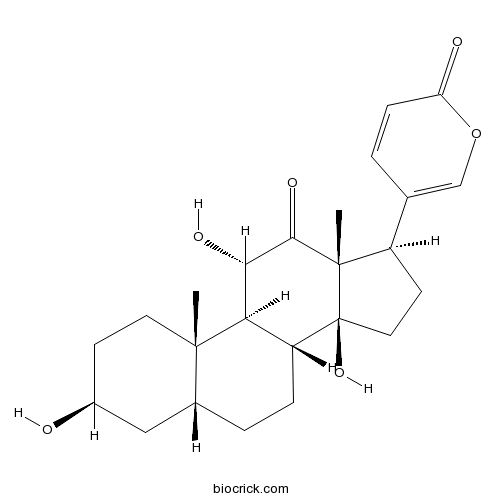
-
BCN2358
Gamabufotalin465-11-2
Instructions
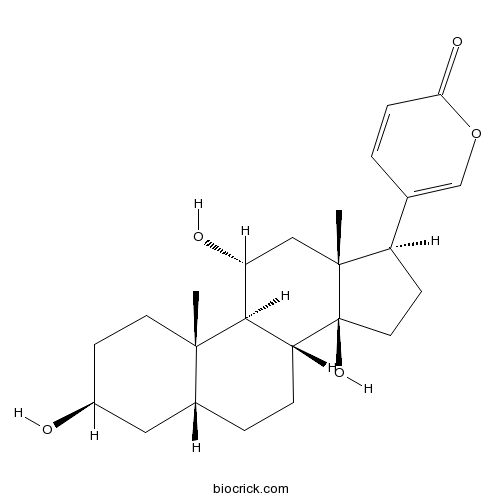
-
BCN1046
Bufalin465-21-4
Instructions
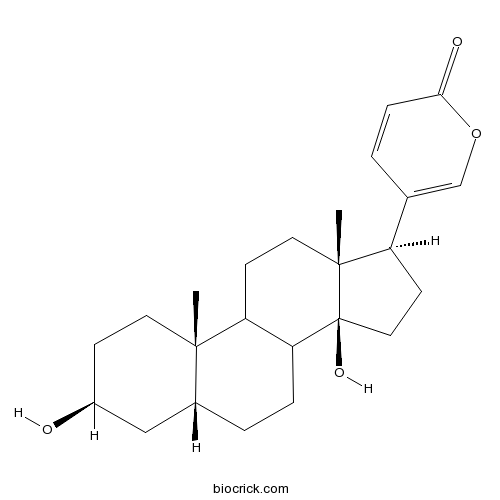
-
BCN5366
Resibufogenin465-39-4
Instructions
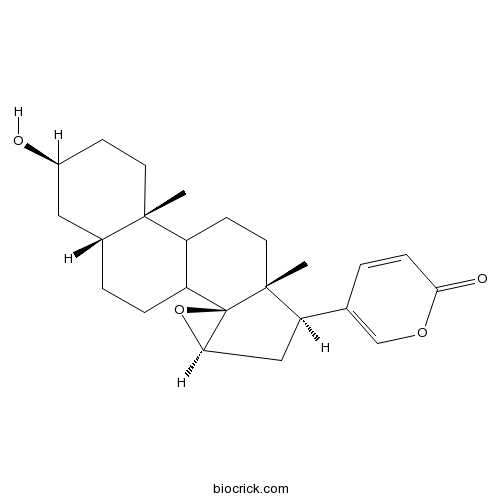
-
BCN8857
Hellebrigenin465-90-7
Instructions

-
BCN5367
Cinobufagin470-37-1
Instructions
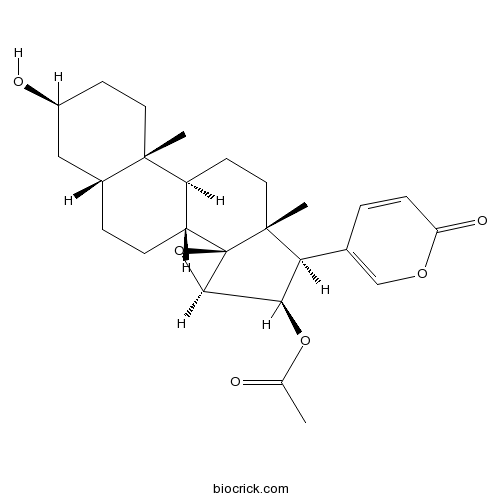
-
BCN5368
Bufotaline471-95-4
Instructions
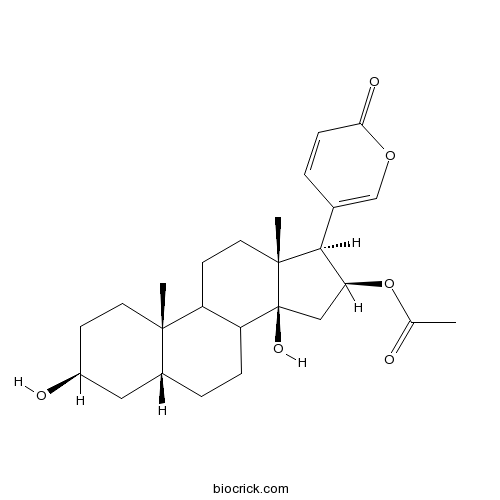
-
BCN2359
Telocinobufagin472-26-4
Instructions
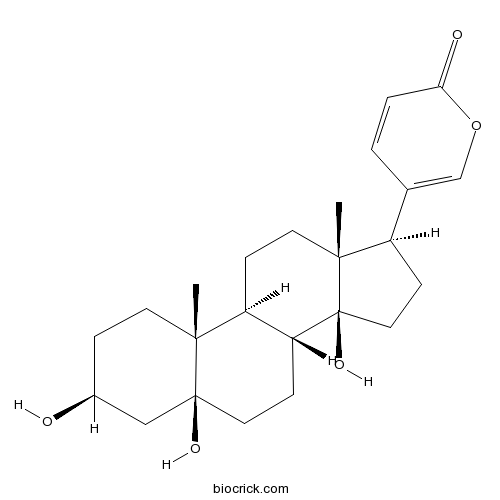
-
BCN8238
Hellebrigenol508-79-2
Instructions
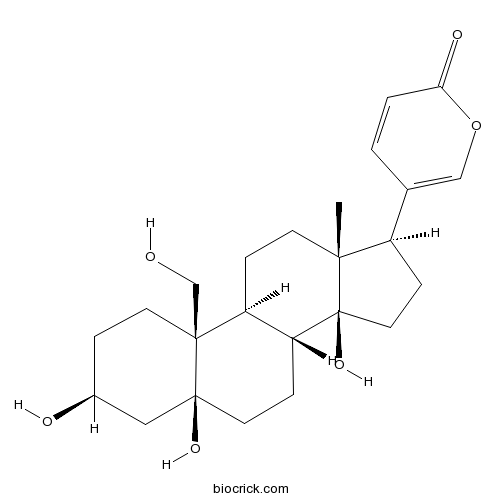
-
BCN2199
Cholesterol57-88-5
Instructions
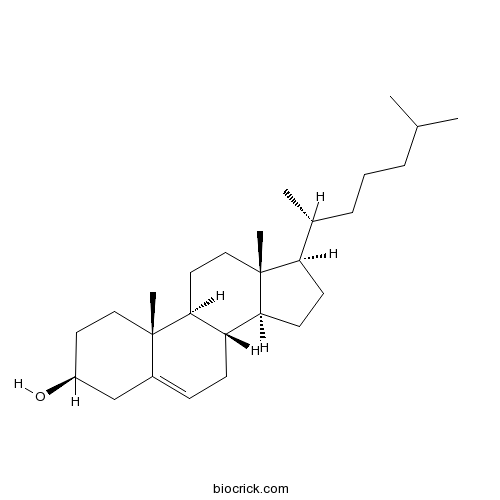
-
BCN8593
Cinobufaginol6691-83-4
Instructions

Transcriptome analyses reveal molecular mechanisms that regulate endochondral ossification in amphibian Bufo gargarizans during metamorphosis.[Pubmed: 30076880]
A developmental transition from aquatic to terrestrial existence is one of the most important events in the evolution of terrestrial vertebrates. Amphibian metamorphosis is a classic model to study this transition. The development of the vertebrate skeleton can reflect its evolutionary history. Endochondral ossification serves a vital role in skeletal development. Thus, we sought to unravel molecular mechanisms that regulate endochondral ossification during Bufo gargarizans metamorphosis.
Transcriptomics provides mechanistic indicators of fluoride toxicology on endochondral ossification in the hind limb of Bufo gargarizans.[Pubmed: 29908452]
None
Identification of <10 KD peptides in the water extraction of Venenum Bufonis from Bufo gargarizans using Nano LC-MS/MS and De novo sequencing.[Pubmed: 29800903]
Skins of anurans (frogs and toads) are rich sources of bioactive peptides. However, the peptides secreted by the skin glands of Bufo gargarizans, the most common toad in China, remained unexplored to date. Here, a strategy combines LC-MS/MS, RNA sequencing and bioinformation analysis was applied to unravel the peptides in the Bufo gargarizans secretions. Data-dependent LC-MS/MS acquisitions of intact peptides followed by automated chromatographic alignment, De novo analysis, database and homology searches with manual validations showed that the venom is composed by 939 features, with masses ranging from 0.7-4 kDa. These peptides derived from 85 proteins were identified using the PEAKS software with acquired MS and MS/MS spectra of Venenum Bufonis against the house-built protein database using De novo RNA sequencing, while only 23 peptides from 8 proteins were found when searching known amphibian database. Moreover, it was found that many peptides with high abundance in Venenum Bufonis derived from proteolytic processing of a larger precursor protein, named as CL4590. Molecular cloning was applied to validate a short domain of CL4590 to evident the accuracy of these obtained sequences. Although the bioactivities of peptides identified by MS/MS are unknown, the next function annotation showed that they may involve in the cell killing, immune and metabolic process, antioxidant activity and antimicrobial actions. Therefore, the peptidomics analysis on Bufo gargarizans discover abundant novel toad peptides which broaden our horizons on the secretion multiplicity and supplied an assortment of pharmacological candidates.
Changes in intestinal microbiota of Bufo gargarizans and its association with body weight during metamorphosis.[Pubmed: 29748695]
The assembly of intestinal microbial communities can play major roles in animal development. We hypothesized that intestinal microbial communities could mirror the developmental programs of amphibian metamorphosis. Here, we surveyed the morphological parameters of the body and intestine of Bufo gargarizans at varying developmental stages and inventoried the intestinal microbial communities of B. gargarizans at four key developmental stages via 16S rDNA gene sequencing. Firstly, our survey showed that during metamorphosis, body weight and intestinal weight were reduced by 56.8 and 91.8%, respectively. Secondly, the gut bacterial diversity of B. gargarizans decreased with metamorphosis and the composition of the tadpoles' intestinal microbiota varied across metamorphosis. Compared to aquatic larvae, terrestrial juveniles showed major shifts in microbial composition, including reduction in Proteobacteria and Actinobacteria, increases in Bacteroidetes and Fusobacteria, and the appearance of Verrucomicrobia. Firmicutes in four developmental stages showed similar abundance at the phylum level, but in each stage was driven by distinct genera. Enterobacter, Aeromonas, Mucinivorans and Bacteroides also changed in abundance and were found to be significantly correlated with loss of body or intestinal tissue during metamorphosis. These results indicate a shift in intestinal microbial community composition throughout amphibian metamorphosis.
Morphological and molecular evidence for a new species of the genus Cosmocercoides Wilkie, 1930 (Ascaridida: Cosmocercidae) from the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura).[Pubmed: 29680942]
A new cosmocercid species, Cosmocercoides qingtianensis sp. n., collected from the intestine of the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura) is described using integrated approaches, including light and scanning electron microscopy, and sequencing and analyzing the ribosomal [small ribosomal DNA (18S) and internal transcribed spacer (ITS)] and mitochondrial [cytochrome c oxidase subunit 1 (cox1)] target regions, respectively. The new species can be distinguished from its congeners by the combination of the following morphological characters, including the large body size, the presence of lateral alae and somatic papillae in both sexes, the length of spicules, the particular morphology and length of gubernaculum, the number, arrangement and morphology of caudal rosettes, the presence of large medioventral precloacal papilla and the long tail. Our molecular analysis revealed the level of intraspecific genetic variation of C. qingtianensis sp. n. distinctly lower than that of the interspecific genetic variation in the ITS and cox1 regions. However, there are some overlaps in the range of intra- and interspecific 18S sequence divergence between the new species and some closely related species. The results of molecular analysis supported the validity of the new species based on the morphological observations. The 18S, ITS, and cox1 regions of C. pulcher collected from Bufo japonicus formosus in Japan were also sequenced and analyzed. The results showed a low level of intraspecific genetic variation in 18S and ITS regions (0-0.12% and 0-0.23% nucleotide differences, respectively), but a relatively high level of intraspecific genetic variation in cox1 region (0.78-4.69% nucleotide differences). In addition, it seems more powerful and practical to use the cox1 region as a genetic marker for the accurate identification and differentiation of species of Cosmocercoides than the 18S and ITS regions, especially for the closely related species.
First report on cystacanths of Sphaerirostris lanceoides (Petrochenko, 1949) (Acanthocephala: Centrorhynchidae) from the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura) in China.[Pubmed: 29637423]
Everted cystacanths of Sphaerirostris lanceoides (Petrochenko, 1949) Golvan 1956 are reported from the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura) for the first time. The prevalence was 1.96% and the intensity ranged between 1.0-3.0 acanthocephalans. SEM observations revealed the morphology of the gonopore and the presence of a flat, bare region on the apical part of the proboscis. Moreover, S. lanceoides was characterised using molecular approaches by sequencing the ribosomal ITS1-5.8S-ITS2 region and the mitochondrial cox1 gene. The resulting ITS sequences were identical and the cox1 sequences showed a divergence of 0-0.75%. Sphaerirostris lanceoides is the first species of the genus for which the ITS1-5.8S-ITS2 and cox1 loci have been sequenced to aid species identification.


