Ginkgo biloba
Ginkgo biloba
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
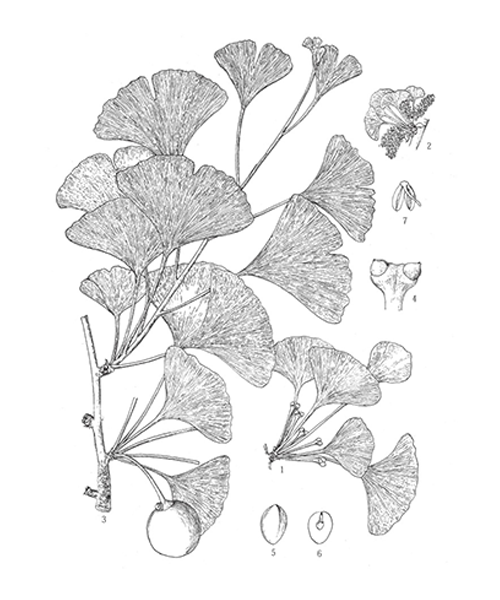
Natural products/compounds from Ginkgo biloba
- Cat.No. Product Name CAS Number COA
-
BCN5939
Ginkgolide J107438-79-9
Instructions

-
BCN5334
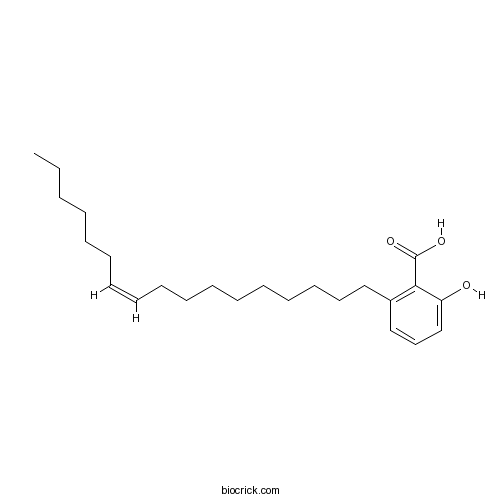
Ginkgolic acid C17:1111047-30-4
Instructions
-
BCC8952
Docosanoic acid112-85-6
Instructions

-
BCN6049
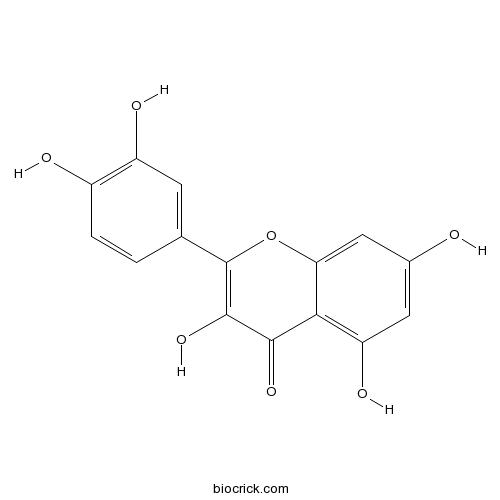
Quercetin117-39-5
Instructions
-
BCN9084
(-)-Sesamin13079-95-3
Instructions

-
BCN1673
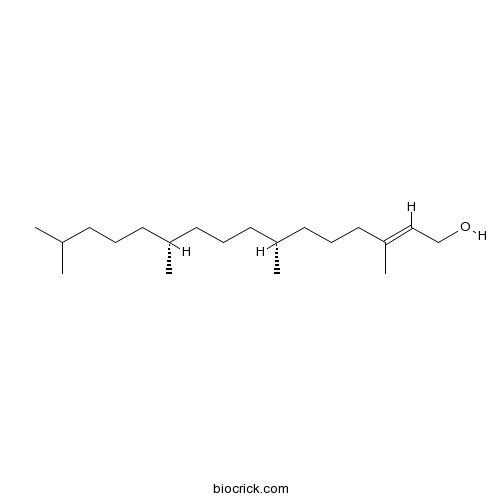
Phytol150-86-7
Instructions
-
BCN2294
Sophoricoside152-95-4
Instructions

-
BCN1680
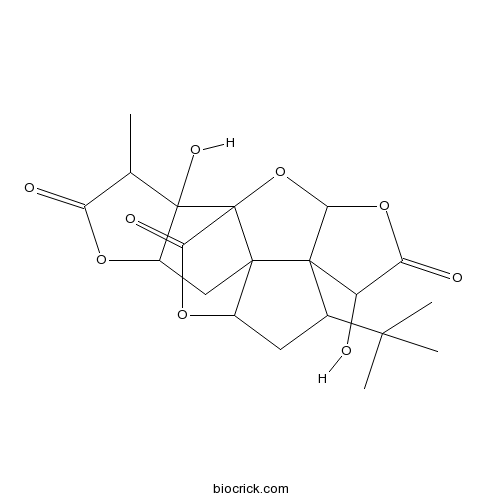
Ginkgolide A15291-75-5
Instructions
-
BCN1681
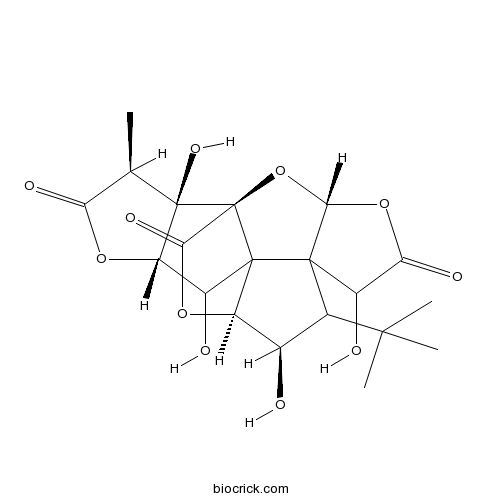
Ginkgolide C15291-76-6
Instructions
-
BCN1682
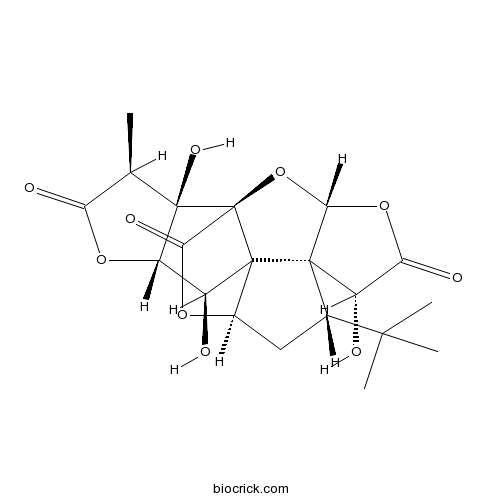
Ginkgolide B15291-77-7
Instructions
-
BCN1684
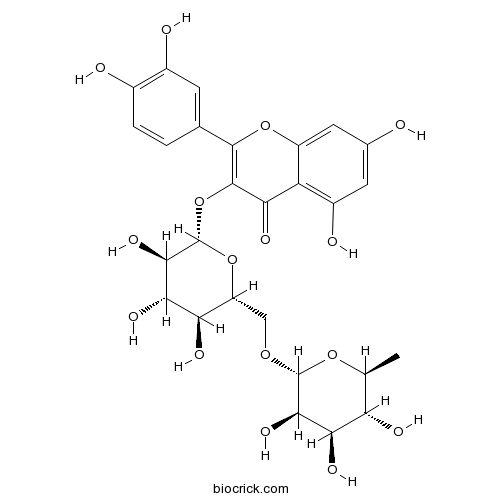
Rutin153-18-4
Instructions
-
BCN6283
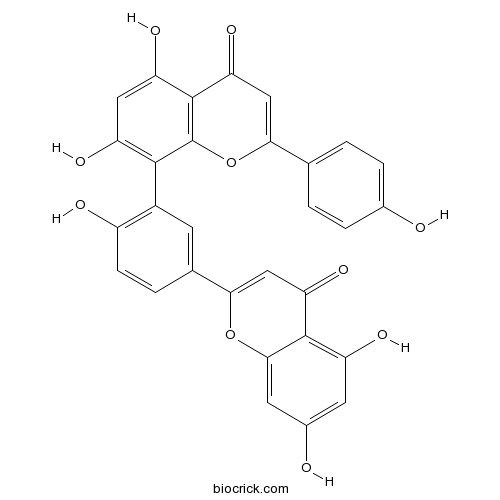
Amentoflavone1617-53-4
Instructions
-
BCN1126
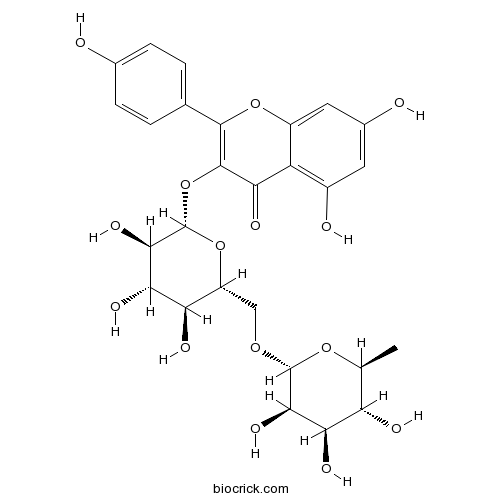
Nicotiflorin17650-84-9
Instructions
-
BCN1520
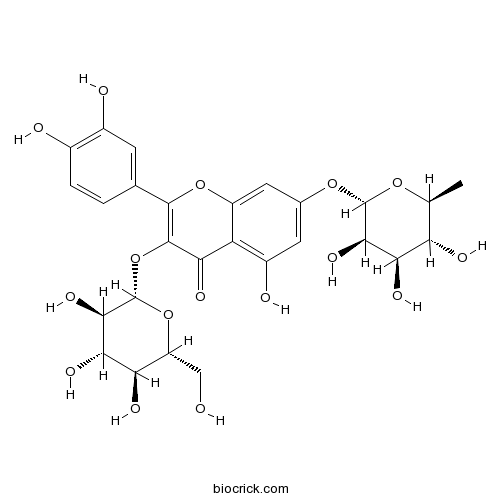
Quercetin 3-O-glucoside-7-O-rhamnoside18016-58-5
Instructions
-
BCN5333
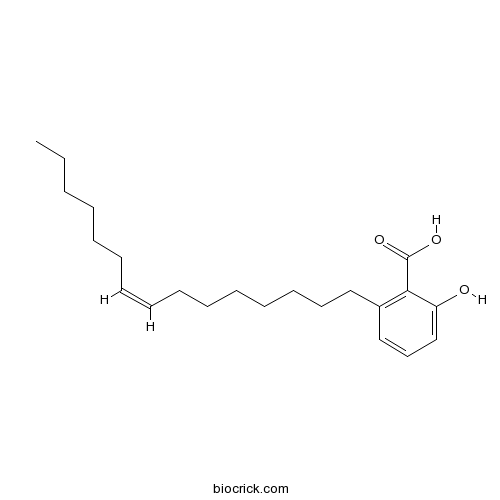
Ginkgolic acid C13:020261-38-5
Instructions

-
BCN2307
Ginkgolic acid C15:122910-60-7
Instructions
-
BCN4081
Cycloolivil3064-05-9
Instructions
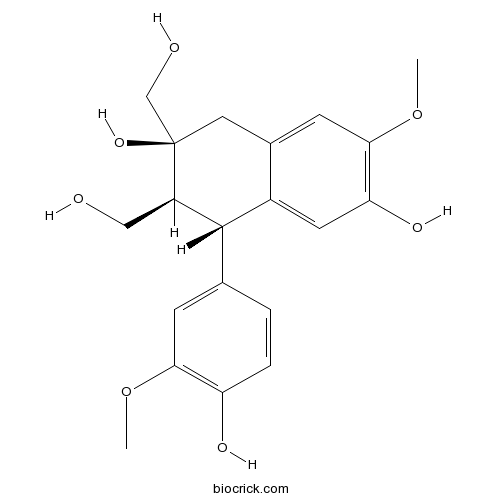
-
BCN1279
(-)-Bilobalide33570-04-6
Instructions
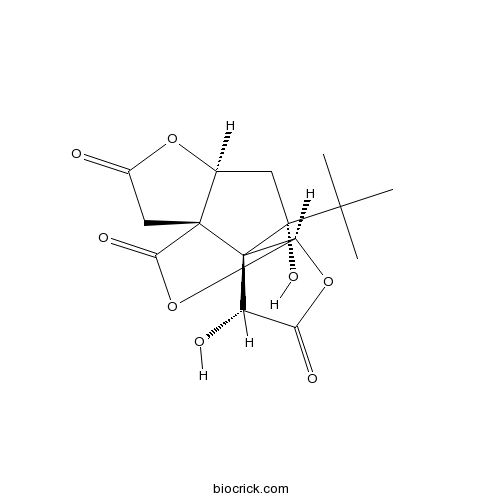
-
BCN5488
Genkwanin437-64-9
Instructions

-
BCN5531
Daucosterol474-58-8
Instructions
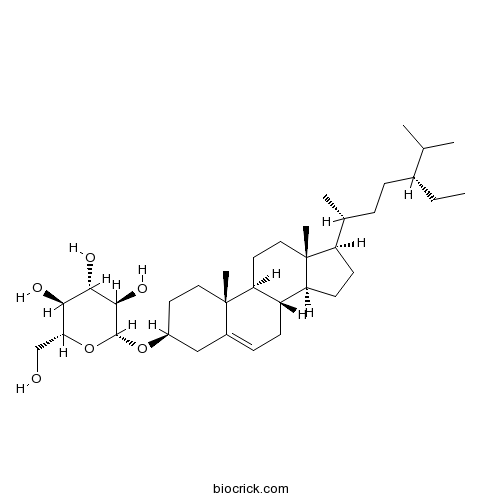
-
BCN5549
Astragalin480-10-4
Instructions

-
BCN5551
Isorhamnetin480-19-3
Instructions

-
BCN5560
Acacetin480-44-4
Instructions

-
BCN2319
Ginkgetin481-46-9
Instructions
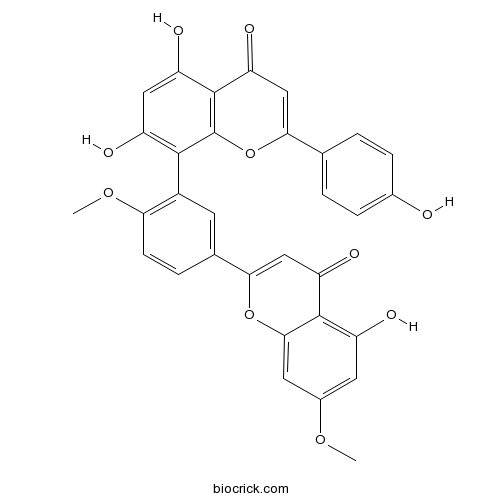
-
BCN5569
Isoquercitrin482-35-9
Instructions
-
BCN5653
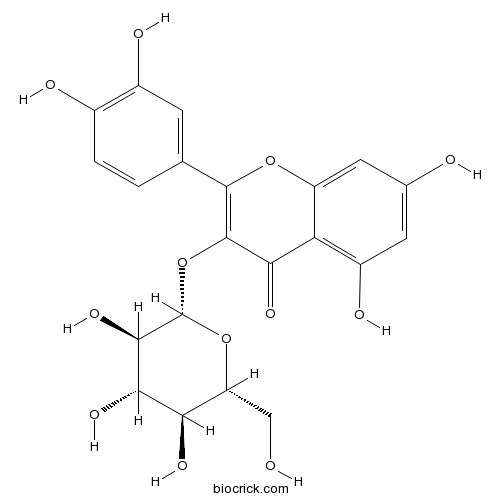
Kaempferol520-18-3
Instructions
-
BCN5658
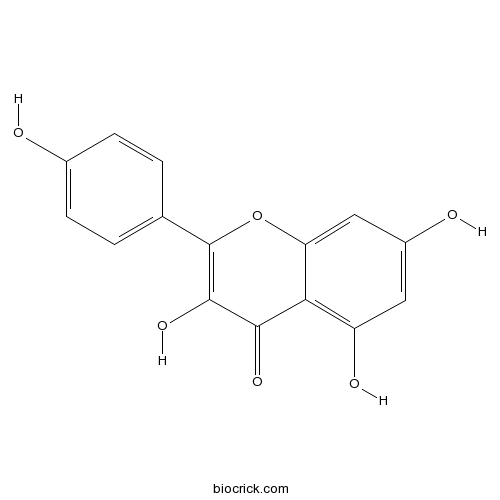
Apigenin520-36-5
Instructions

-
BCN5661
Bilobetin521-32-4
Instructions

-
BCN5662
Sciadopitysin521-34-6
Instructions

-
BCN5665
Quercitrin522-12-3
Instructions
-
BCN2320
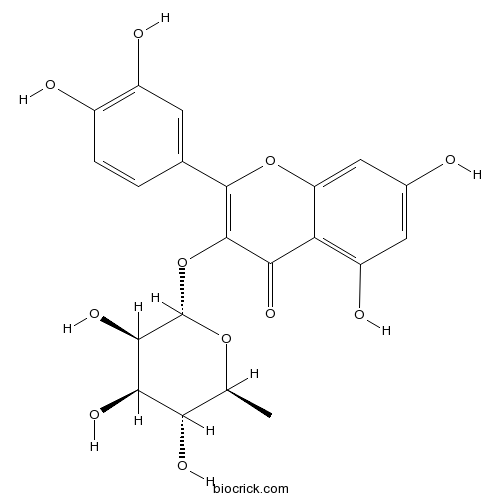
Isoginkgetin548-19-6
Instructions
-
BCN2973
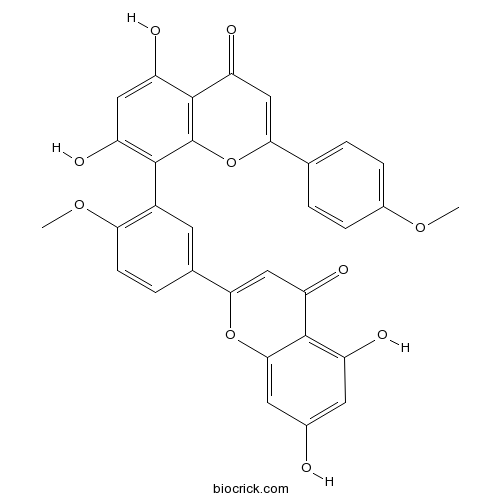
1-Octacosanol557-61-9
Instructions

-
BCN1010
D-(+)-Xylose58-86-6
Instructions

-
BCN1263
Narcissoside604-80-8
Instructions

Standardized Ginkgo biloba extract EGb 761® attenuates early brain injury following subarachnoid hemorrhage via suppressing neuronal apoptosis through the activation of Akt signaling.[Pubmed: 30098550]
Early brain injury (EBI) plays a critical role in determining the outcome of subarachnoid hemorrhage (SAH). The present study was designed to investigate the role of EGb 761, a standardized extract of Ginkgo biloba, in SAH-induced EBI and to explore its potential mechanism of action.
Effects of ginkgo biloba extract on the cognitive function and expression profile of inflammatory factors in a rat model of hemorrhagic stroke.[Pubmed: 30096131]
Hemorrhagic stroke is a major risk factor for cognitive impairment. Our study aimed to measure the effect of ginkgo biloba extract (EGB761) on the cognitive ability and inflammatory expression in hemorrhagic stroke model SD rats and to analyze their relationship. Forty SD rats were divided randomly into an SD group (normal control SD rats), an SD+EGB761 group (normal control SD rats supplemented with 45 mg/kg EGB761), a CO group (hemorrhagic stroke model SD rats using collagenase), and a CO+EGB761 group (hemorrhagic stroke model SD rats supplemented with 45 mg/kg EGB761) consisting of 10 rats, respectively. The Y-electric maze test was selected to measure the cognitive function in four groups. Furthermore, enzyme-linked immunosorbent assay and real-time PCR were, respectively, applied for detecting the protein and gene expression profiles of inflammatory factors in primary cultured microglia. Compared with rats in the SD group, the average time of electrical simulation for mastering criteria was prolonged in the CO group (P<0.05). Furthermore, expression levels of proinflammatory cytokines interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α and anti-inflammatory cytokines IL-4, IL-10, and tumor necrosis factor-β were significantly increased and decreased, respectively, in rats of the CO group compared with the SD group (P<0.05). The results of electrical simulation time, inflammatory factors protein, and gene expression profile in rats of the CO+EGB761 group compared with the CO group were opposite to above contrast (P<0.05). Ginkgo biloba extract could alleviate the cognitive dysfunction after hemorrhagic stroke in SD rats; this is associated with regulating the expression of inflammatory factors secreted by microglia.
A possible new mechanism and drug intervention for kidney damage due to arsenic poisoning in rats.[Pubmed: 30090365]
Arsenic poisoning is a worldwide endemic disease that affects thousands of people. Currently, the aetiology of the disease is known, but its pathogenesis is uncharacterized and there is no specific treatment. We established a rat model of coal-burning arsenic poisoning by feeding the animals corn powder baked with high arsenic coal. By observing subsequent changes in kidney and immune function, we found that arsenic induces both kidney and immune damage. Furthermore, there is a significant correlation between kidney and immune damage. Moreover, Ginkgo biloba, a known immune enhancer, was used as an intervention agent in arsenic poisoned rats to validate the relationship between kidney and immune damage. Meanwhile, we also explored the mechanism of Ginkgo biloba treatment of kidney damage in burning-coal arsenic poisoned rats. We found that Ginkgo biloba enhanced immune function in rats with arsenic poisoning and ameliorated arsenic-induced kidney damage. These results suggest that immune suppression may be one of the mechanisms underlying arsenic-induced kidney damage and that Ginkgo biloba might relieve kidney damage by enhancing immune function.
Two new phenolic glycosides isolated from Ginkgo seeds.[Pubmed: 30080649]
Two new phenolic glycosides, 7S, 8R-urolignoside-9'-O-β-D-glucoside (1) and scrophenoside G (2), were isolated and identified from the seeds of Ginkgo biloba L., a famous traditional medicine and functional food around the world. Their structures were elucidated by spectroscopic methods (1D and 2D NMR, HR-ESI-MS, and CD), and the comparisons of spectroscopic data with the reported values in the literature.
Chemical analysis, pharmacological activity and process optimization of the proportion of bilobalide and ginkgolides in Ginkgo biloba extract.[Pubmed: 30071391]
Variations on the efficacy of commercial Ginkgo biloba preparations have been reported, although all the products follow the same standards. Terpene trilactones (TTLs), including bilobalide (BB) and ginkgolides, are one of the main active components in G. biloba extract and have been received the most attention due to their chemical uniqueness and their importance for quality control. A plenty of studies demonstrated that BB and ginkgolides display differential activities on various biological processes. However, the influence of different ratios of BB and ginkgolides on the efficacy of TTLs has not been detected yet. The aims of this study were: (1) to test whether different ratios of BB and ginkgolides existed in commercial G. biloba preparations; (2) to detect the influence of different ratios of BB and ginkgolides on the in vivo efficacy of TTLs; and (3) to optimize the extraction process of G. biloba to approach the better BB and ginkgolides ratio with the maximum in vivo effects. First, the content and proportion of BB and ginkgolides in various G. biloba preparations were quantified by HPLC-MS analysis. As the results, an obvious fluctuation in the proportion of BB and ginkgolides was observed in the preparations from different commercial suppliers. The ratio was ranged from 0.3 to 0.8. Second, a zebrafish thrombosis model was used to evaluate the antithrombotic effects of different ratios of BB and ginkgolides. The result showed that the proportion of BB and ginkgolides at 1:2 produced the maximum antithrombotic effects. Third, the extraction process of G. biloba was optimized using a design space technique aiming to approach the best BB and ginkgolides ratio obtained from zebrafish experiment. The extraction process was modeled based on the results of Box-Behnken designed experiments. Design space was then calculated using a probability-based method. Within this design space, G. biloba extraction process can be guaranteed to achieve the better BB and ginkgolides ratio with high assurance. Normal operation space for G. biloba extraction process was recommended as ethanol concentration of 50% to 70%, liquid-to-solid ratio of 5.6 mL/g to 7.3 mL/g, and extraction time of 2.2 h to 3.0 h. This work not only suggest that the proportion of BB and ginkgolides should be used as a quality control index in ginkgo preparations besides the content of TTLs, but also provide a way to approach it with the extraction process parameters controlled in the normal operation ranges.
Human pharmacokinetics of ginkgo terpene lactones and impact of carboxylation in blood on their platelet-activating factor antagonistic activity.[Pubmed: 30054600]
Terpene lactones are a class of bioactive constituents of standardized preparations of Ginkgo biloba leaf extract, extensively used as add-on therapies in patients with ischemic cardiovascular and cerebrovascular diseases. This investigation evaluated human pharmacokinetics of ginkgo terpene lactones and impact of their carboxylation in blood. Human subjects received oral YinXing-TongZhi tablet or intravenous ShuXueNing, two standardized ginkgo preparations. Their plasma protein-binding and platelet-activating factor antagonistic activity were assessed in vitro. Their carboxylation was assessed in phosphate-buffered saline (pH 7.4) and in human plasma. After dosing YinXing-TongZhi tablet, ginkgolides A and B and bilobalide exhibited significantly higher systemic exposure levels than ginkgolides C and J; after dosing ShuXueNing, ginkgolides A, B, C, and J exhibited high exposure levels. The compounds' unbound fractions in plasma were 45-92%. Apparent oral bioavailability of ginkgolides A and B was mostly >100%, while that of ginkgolides C and J was 6-15%. Bilobalide's bioavailability was probably high but lower than that of ginkgolides A/B. Terminal half-lives of ginkgolides A, B, and C (4-7 h) after dosing ShuXueNing were shorter than their respective values (6-13 h) after dosing YinXing-TongZhi tablet. Half-life of bilobalide after dosing the tablet was around 5 h. Terpene lactones were roughly evenly distributed in various body fluids and tissues; glomerular-filtration-based renal excretion was the predominant elimination route for the ginkgolides and a major route for bilobalide. Terpene lactones circulated as trilactones and monocarboxylates. Carboxylation reduced platelet-activating factor antagonistic activity of ginkgolides A, B, and C. Ginkgolide J, bilobalide, and ginkgo flavonoids exhibited no such bioactivity. Collectively, differences in terpene lactones' exposure between the two preparations and influence of their carboxylation in blood should be considered in investigating the relative contributions of terpene lactones to ginkgo preparations' therapeutic effects. The results here will inform rational clinical use of ginkgo preparations.
Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat.[Pubmed: 30030129]
Perinatal hypoxic-ischemic (HI) insult is an important cause of brain injury in neonates. The development of novel treatment strategies for neonates with HI brain injury is urgently needed. Ginkgolide B (GB) is a main component of Ginkgo biloba extracts with a long history of use in traditional Chinese medicine. However, it is unknown whether GB could play a protective role in hypoxic stress in immature animals.


