(-)-BilobalideNeuroprotective agent CAS# 33570-04-6 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33570-04-6 | SDF | Download SDF |
| PubChem ID | 73581 | Appearance | White powder |
| Formula | C15H18O8 | M.Wt | 326.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | (-)-Bilobalide | ||
| Solubility | DMSO : ≥ 100 mg/mL (306.47 mM) *"≥" means soluble, but saturation unknown. | ||
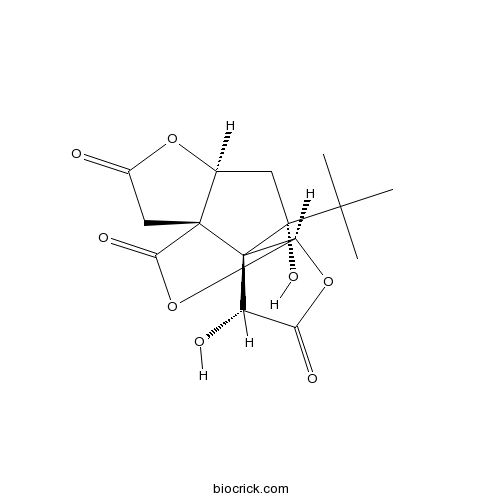
| SMILES | CC(C)(C)C1(CC2C3(C14C(C(=O)OC4OC3=O)O)CC(=O)O2)O | ||
| Standard InChIKey | MOLPUWBMSBJXER-YDGSQGCISA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bilobalide possesses anticonvulsant, insecticidal, and cardioprotective effects. Bilobalide exerts protective and trophic effects on neurons, the PI3K/Akt pathway may be involved in the protective effects of bilobalide; it also can protect PC12 cells from A beta 25-35-induced cytotoxicity, it dose-dependently attenuates the cytotoxic effect of A beta 25-35. |
| Targets | PAFR | Beta Amyloid | PI3K | Akt | ERK | PKC | Serine |
| In vitro | Protective effects of bilobalide on amyloid beta-peptide 25-35-induced PC12 cell cytotoxicity.[Pubmed: 11263252]Acta Pharmacol Sin. 2000 Jan;21(1):75-9.To study the effect of bilobalide[(-)-Bilobalide], a terpene extracted from the leaves of Ginkgo biloba, on beta-amyloid peptide fragment 25-35 (A beta 25-35)-induced PC12 cell cytotoxicity. |
| In vivo | Effects of bilobalide on gamma-aminobutyric acid levels and glutamic acid decarboxylase in mouse brain.[Pubmed: 10078989]Eur J Pharmacol. 1999 Feb 19;367(2-3):165-73.We have previously demonstrated that Bilobalide[(-)-Bilobalide] a constituent of the Ginkgo biloba extract, possesses anticonvulsant activity, and suggested that the mechanism of its anticonvulsant action involves modulation of y-aminobutyric acid (GABA)-related neuronal transmission. |
| Kinase Assay | Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells.[Pubmed: 20333467 ]Apoptosis. 2010 Jun;15(6):715-27.Bilobalide[(-)-Bilobalide], a sesquiterpene trilactone constituent of Ginkgo biloba leaf extracts, has been proposed to exert protective and trophic effects on neurons. However, mechanisms underlying the protective effects of bilobalide [(-)-Bilobalide]remain unclear. Using human SH-SY5Y neuroblastoma cells and primary hippocampal neurons, this study investigated the neuroprotective effects of bilobalide[(-)-Bilobalide]. |
| Cell Research | Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor[Pubmed: 21391918]Biosci Rep. 2011 Oct 1; 31(Pt 5): 439–447.Terpene trilactones from Ginkgo biloba have been investigated extensively for their antioxidant and anti-ischaemic activities on the brain and the heart, but the mechanisms of these effects remain unclear. |
| Structure Identification | The Korean Society of Pesticide Science,2001,5(1):24-9.Insecticidal Activities of Bilobalide from Ginkgo biloba Leaves and its Derivatives.[Reference: WebLink]This study was conducted to investigate insecticidal activities of Ginkgo biloba (L.) leaves-derived bilobalide[(-)-Bilobalide] and its hydrolysis and oxidation products against adults of Nilaparavata lugens Stal. |

(-)-Bilobalide Dilution Calculator

(-)-Bilobalide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0647 mL | 15.3233 mL | 30.6466 mL | 61.2933 mL | 76.6166 mL |
| 5 mM | 0.6129 mL | 3.0647 mL | 6.1293 mL | 12.2587 mL | 15.3233 mL |
| 10 mM | 0.3065 mL | 1.5323 mL | 3.0647 mL | 6.1293 mL | 7.6617 mL |
| 50 mM | 0.0613 mL | 0.3065 mL | 0.6129 mL | 1.2259 mL | 1.5323 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3065 mL | 0.6129 mL | 0.7662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 3.33 (pIC50 Value) [1] Bilobalide is a biologically active terpenic trilactone present in Ginkgo biloba. An increasing number of studies have demonstrated its neuroprotective effects. in vitro: Inhibition by BB and GB was abolished in mutant receptors containing T6'S and S12'A substitutions, but their potencies were enhanced (42- and 125-fold, respectively) in S2'A mutant receptors [1]. BB enhanced the secretion of α-secretase-cleaved soluble amyloid precursor protein (sAPPα, a by-product of non-amyloidogenic processing of APP) and decreased the β amyloid protein (Aβ, a by-product of amyloidogenic processing of APP) via PI3K-dependent pathway [2]. in vivo: Oral administration of bilobalide (10-30 mg/kg) significantly inhibited thermal hyperalgesia in response to carrageenan, capsaicin and paw incision, independent of dose, with an efficacy similar to that of diclofenac. In the carrageenan model, mechanical hypersensitivity and paw oedema were also significantly reduced after treatment with bilobalide (10-30 mg/kg) [3]. BB(4 and 8 mg/kg) significantly protected VD rats against cognitive deficits in the Morris water maze. Biochemical assessment showed that BB (4 and 8 mg/kg) increased superoxide dismutase (SOD) activity and glutathione (GSH) content, and decreased nitric oxide synthase (NOS) activity and malondialdehyde (MDA) content [4]. Clinical trial: N/A
- Cyclo(Phe-Leu)
Catalog No.:BCN2418
CAS No.:3354-31-2
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- (S)-HexylHIBO
Catalog No.:BCC7167
CAS No.:334887-48-8
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
- 19,20-(E)-Vallesamine
Catalog No.:BCN5260
CAS No.:3368-87-4
Protective effects of bilobalide on amyloid beta-peptide 25-35-induced PC12 cell cytotoxicity.[Pubmed:11263252]
Acta Pharmacol Sin. 2000 Jan;21(1):75-9.
AIM: To study the effect of bilobalide, a terpene extracted from the leaves of Ginkgo biloba, on beta-amyloid peptide fragment 25-35 (A beta 25-35)-induced PC12 cell cytotoxicity. METHODS: 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide and lactate dehydrogenase assay were used to measure the viability of PC12 cells. Thiobarbituric acid-reactive substances were measured to determine lipid peroxidation of cells. Antioxidant enzymes in PC12 cells were detected. RESULTS: Treatment of PC12 cells with A beta 25-35 (100 mumol.L-1) for 24 h caused a great decrease in cell viability (P < 0.01 compared with control). Bilobalide 25-100 mumol.L-1 dose-dependently attenuated the cytotoxic effect of A beta 25-35. Bilobalide also inhibited A beta 25-35 (100 mumol.L-1)-induced elevation of lipid peroxidation and decline of antioxidant enzyme activities. CONCLUSION: Bilobalide protected PC12 cells from A beta 25-35-induced cytotoxicity.
Effects of bilobalide on gamma-aminobutyric acid levels and glutamic acid decarboxylase in mouse brain.[Pubmed:10078989]
Eur J Pharmacol. 1999 Feb 19;367(2-3):165-73.
We have previously demonstrated that bilobalide, a constituent of the Ginkgo biloba extract, possesses anticonvulsant activity, and suggested that the mechanism of its anticonvulsant action involves modulation of y-aminobutyric acid (GABA)-related neuronal transmission. This study examined the effects of bilobalide on the level of GABA and glutamate, the activity and the amount of glutamic acid decarboxylase (EC 4.1.1.15), and the function of GABA(A) receptors in the hippocampus, cerebral cortex and striatum of the mouse. GABA levels, glutamic acid decarboxylase activity, and the protein amount of 67 kDa glutamic acid decarboxylase in the hippocampus of mice treated with bilobalide (30 mg/kg, p.o., once a day for 4 days) were significantly higher than those in controls. However, there were no significant differences in glutamate levels or, the number and the dissociation constants of GABA(A) receptors in the hippocampus between control and bilobalide-treated mice. These results suggest that the anticonvulsant effect of bilobalide is due to elevation of GABA levels, possibly through potentiation of glutamic acid decarboxylase activity and enhancement of the protein amount of 67 kDa glutamic acid decarboxylase by bilobalide.
Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells.[Pubmed:20333467]
Apoptosis. 2010 Jun;15(6):715-27.
Bilobalide, a sesquiterpene trilactone constituent of Ginkgo biloba leaf extracts, has been proposed to exert protective and trophic effects on neurons. However, mechanisms underlying the protective effects of bilobalide remain unclear. Using human SH-SY5Y neuroblastoma cells and primary hippocampal neurons, this study investigated the neuroprotective effects of bilobalide. We mimicked aging-associated neuronal impairments by applying external factors (beta amyloid protein (Abeta) 1-42, H(2)O(2) and serum deprivation) consequently inducing cell apoptosis. As markers for apoptosis, cell viability, DNA fragmentation, mitochondrial membrane potential and levels of cleaved caspase 3 were measured. We found that, bilobalide prevented Abeta 1-42-, H(2)O(2)- and serum deprivation-induced apoptosis. To better understand the neuroprotective effects of bilobalide, we also tested the ability of bilobalide to modulate pro-survival signaling pathways such as protein kinase C (PKC), extracellular-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways. It was found that, bilobalide dose-dependently increased PI3K activity and levels of phosphorylated Akt (p-Akt Ser473 and Thr308), which could be maintained up to at least 2 h after bilobalide withdrawal in cells treated with or without Abeta 1-42, H(2)O(2) or serum-free medium. In addition, application of PI3K/Akt inhibitor LY294002 could abrogate both the protective effects of bilobalide against Abeta 1-42-, H(2)O(2)- and serum deprivation-induced apoptotic cell damage and bilobalide-induced increase in PI3K activity and levels of p-Akt (Ser473 and Thr308). In contrast, application of PKC inhibitor staurosporine (STS) did not affect the protective effects of bilobalide. Moreover, no change in levels of phosphorylated ERK1/2 (p-ERK1/2) was observed in bilobalide-treated cells. These results further suggested that the PI3K/Akt pathway might be involved in the protective effects of bilobalide. Since modern technology allows production of purified bilobalide with high bioavailability, bilobalide may be useful in developing therapy for diseases involving age-associated neurodegeneration.
Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor.[Pubmed:21391918]
Biosci Rep. 2011 Oct;31(5):439-47.
Terpene trilactones from Ginkgo biloba have been investigated extensively for their antioxidant and anti-ischaemic activities on the brain and the heart, but the mechanisms of these effects remain unclear. For the present study, a terpenoid constituent from G. biloba, bilobalide, was screened for protective effects on the ischaemic heart and the involvement of the PAFR [PAF (platelet-activating factor) receptor] and the enzyme that degrades PAF, PAF-AH (PAF acetylhydrolase) during hypoxia. The PAF pathway is supposed to play a role in hypoxia and its regulation may prevent or alleviate MI (myocardial infarction). Cardiomyocytes from neonatal rat hearts were cultured and treated with different concentrations of bilobalide (500-0.5 ng/ml). After being subjected to a hypoxic environment, the cells' viability was evaluated and proteins as well as RNA were extracted for analysis by Western blotting and RT-PCR (reverse transcription PCR) respectively. With the MI model we tested for bilobalide's cardioprotective effects and the involvement of PAFR and PAF-AH. Bilobalide (5 ng/ml) significantly decreased the mortality of cells in a concentration-dependent way. mRNA expression of PAFR was up-regulated in hypoxic cells but in the groups treated with bilobalide, its expression was down-regulated to the level of the normal control. In hypoxic tissue, PAFR protein expression was also up-regulated, but was reduced in the bilobalide (10 mg/kg of body weight) treated group. Our results indicate that PAF and its receptor may be involved in the cellular response of cardiomyocytes to hypoxia and that bilobalide may interact with this receptor to exert its cardioprotective effects.


