BritannilactoneCAS# 33620-72-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33620-72-3 | SDF | Download SDF |
| PubChem ID | 102004712 | Appearance | Cryst. |
| Formula | C15H22O4 | M.Wt | 266.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Desacetylinulicin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
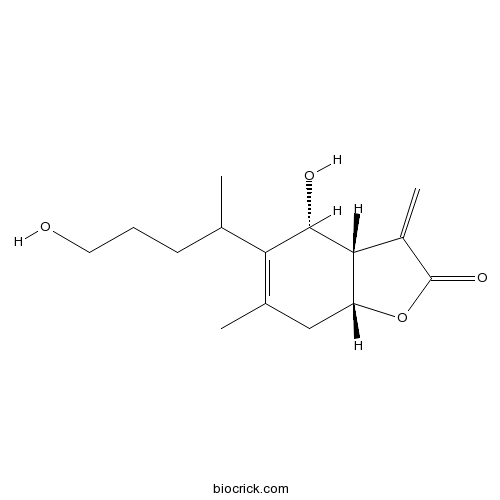
| Chemical Name | (3aS,4R,7aR)-4-hydroxy-5-(5-hydroxypentan-2-yl)-6-methyl-3-methylidene-3a,4,7,7a-tetrahydro-1-benzofuran-2-one | ||
| SMILES | CC1=C(C(C2C(C1)OC(=O)C2=C)O)C(C)CCCO | ||
| Standard InChIKey | ASZIGQFYGXSPCO-COIIJPPFSA-N | ||
| Standard InChI | InChI=1S/C15H22O4/c1-8(5-4-6-16)12-9(2)7-11-13(14(12)17)10(3)15(18)19-11/h8,11,13-14,16-17H,3-7H2,1-2H3/t8?,11-,13-,14+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Britannilactone significantly reduces melanin production in a dose-dependent manner with the IC50 value of 15.5 uM. |

Britannilactone Dilution Calculator

Britannilactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Britannilactone(Desacetylinulicin) is a methanol extract of the dried flower of Inula britannica L. IC50 value: Target: A bioassay-guided isolation of the chloroform fraction of the I. britannica using an in vitro melanogenesis inhibition assay led to the isolation of sesquiterpenes, 1-O-acetylbritannilactone (1), britannilactone (2) and neobritannilactone B (3). Compounds 1 and 2 significantly reduced melanin production in a dose-dependent manner with IC50 values of 13.3 and 15.5 μM, respectively [1].
References:
[1]. Choo SJ, et al. Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells. Arch Pharm Res. 2014 May;37(5):567-74.
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- (-)-Bilobalide
Catalog No.:BCN1279
CAS No.:33570-04-6
- Cyclo(Phe-Leu)
Catalog No.:BCN2418
CAS No.:3354-31-2
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- Desoxyrhapontigenin
Catalog No.:BCN6479
CAS No.:33626-08-3
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
- 19,20-(E)-Vallesamine
Catalog No.:BCN5260
CAS No.:3368-87-4
- Pachypodol
Catalog No.:BCN5261
CAS No.:33708-72-4
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- Voacamine
Catalog No.:BCN8433
CAS No.:3371-85-5
- 4,4'-Di-O-methylellagic acid
Catalog No.:BCN3709
CAS No.:3374-77-4
Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells.[Pubmed:24346861]
Arch Pharm Res. 2014 May;37(5):567-74.
During the course of screens to identify anti-melanogenic agents from natural resources, we found that the methanol extract of the dried flower of Inula britannica L. inhibited melanin synthesis in cultured melanoma cells stimulated with 3-isobutyl-1-methylxanthine (IBMX). A bioassay-guided isolation of the chloroform fraction of the I. britannica using an in vitro melanogenesis inhibition assay led to the isolation of sesquiterpenes, 1-O-acetylBritannilactone (1), Britannilactone (2) and neoBritannilactone B (3). Compounds 1 and 2 significantly reduced melanin production in a dose-dependent manner with IC50 values of 13.3 and 15.5 muM, respectively, whereas compound 3 was found to be cytotoxic. Compound 1 also inhibited the tyrosinase activity only in cell based-systems. Western blot analysis indicated that compound 1 inhibited melanogenesis by activating extracellular signal-regulated kinase (ERK) and Akt signaling and also inhibiting cAMP related binding protein, which regulates its downstream pathway, including tyrosinase, tyrosinase related protein-1 and TRP-2. These results demonstrated that compound 1, a major sesquiterpene from the flowers of I. britannica, exhibited anti-melanogenic activity by suppression of tyrosinase expression via ERK and Akt signaling. Taken together, our results suggest that these compounds may act as potent natural skin-lightening agents.


