DesoxyrhapontigeninCAS# 33626-08-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33626-08-3 | SDF | Download SDF |
| PubChem ID | 6255462 | Appearance | White-beige powder |
| Formula | C15H14O3 | M.Wt | 242.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 3,5-Dihydroxy 4'-methoxy trans-stilbene; 4-Methoxyresveratrol; 4'-Methoxy 3,5-stilbenediol; 4'-O-Methylresveratrol; Resveratrol 4'-methyl ether | ||
| Solubility | Soluble in acetone, acetonitrile, chloroform, dichloromethane, DMSO, ethanol, ethyl acetate and methan | ||
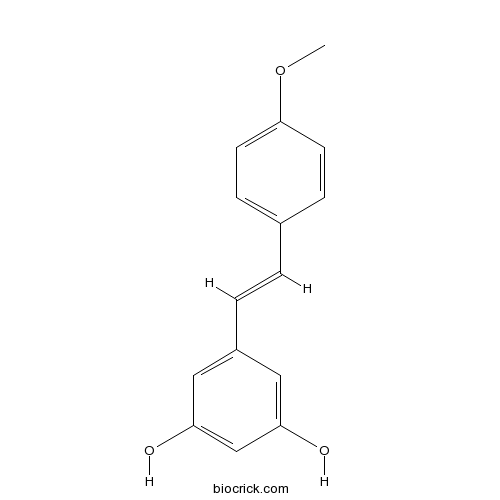
| Chemical Name | 5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene-1,3-diol | ||
| SMILES | COC1=CC=C(C=C1)C=CC2=CC(=CC(=C2)O)O | ||
| Standard InChIKey | IHVRWFJGOIWMGC-NSCUHMNNSA-N | ||
| Standard InChI | InChI=1S/C15H14O3/c1-18-15-6-4-11(5-7-15)2-3-12-8-13(16)10-14(17)9-12/h2-10,16-17H,1H3/b3-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Desoxyrhapontigenin shows significant monoamine oxidase inhibitory activities with the IC50 value of 11.5 ± 1.1 uM. 2. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1(HO-1) expression in macrophages and inflammatory lung injury, and HO-1 is an important anti-inflammatory, antioxidative and cytoprotective enzyme. 3. Desoxyrhapontigenin shows anti-inflammatory properties by the inhibition of iNOS and COX-2 expression via the down-regulation of the MAPK signaling pathways and the inhibition of NF-κB and Akt activation. 4. Desoxyrhapontigenin and desoxyrhaponticin have significant hypoglycemic and antioxidant effects. |
| Targets | Nrf2 | HO-1 | NOS | COX | NF-kB | Akt | MAPK | JNK | SOD | ERK |

Desoxyrhapontigenin Dilution Calculator

Desoxyrhapontigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1271 mL | 20.6356 mL | 41.2712 mL | 82.5423 mL | 103.1779 mL |
| 5 mM | 0.8254 mL | 4.1271 mL | 8.2542 mL | 16.5085 mL | 20.6356 mL |
| 10 mM | 0.4127 mL | 2.0636 mL | 4.1271 mL | 8.2542 mL | 10.3178 mL |
| 50 mM | 0.0825 mL | 0.4127 mL | 0.8254 mL | 1.6508 mL | 2.0636 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4127 mL | 0.8254 mL | 1.0318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Britannilactone
Catalog No.:BCN3509
CAS No.:33620-72-3
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- (-)-Bilobalide
Catalog No.:BCN1279
CAS No.:33570-04-6
- Cyclo(Phe-Leu)
Catalog No.:BCN2418
CAS No.:3354-31-2
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Britannin
Catalog No.:BCN2366
CAS No.:33627-28-0
- 1-O-Acetylbritannilactone
Catalog No.:BCN7715
CAS No.:33627-41-7
- (S)-(+)-Ketamine hydrochloride
Catalog No.:BCC7930
CAS No.:33643-47-9
- H-Thr-Obzl.HCl
Catalog No.:BCC2674
CAS No.:33645-24-8
- Secnidazole
Catalog No.:BCC4971
CAS No.:3366-95-8
- Hypophyllanthin
Catalog No.:BCN5259
CAS No.:33676-00-5
- 19,20-(E)-Vallesamine
Catalog No.:BCN5260
CAS No.:3368-87-4
- Pachypodol
Catalog No.:BCN5261
CAS No.:33708-72-4
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- Voacamine
Catalog No.:BCN8433
CAS No.:3371-85-5
- 4,4'-Di-O-methylellagic acid
Catalog No.:BCN3709
CAS No.:3374-77-4
- Cannabivarin
Catalog No.:BCN7587
CAS No.:33745-21-0
Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury.[Pubmed:24624340]
Redox Biol. 2014 Feb 18;2:504-12.
Heme oxygenase-1 (HO-1) is an important anti-inflammatory, antioxidative and cytoprotective enzyme that is regulated by the activation of the major transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2). In the present study, six stilbene derivatives isolated from Rheum undulatum L. were assessed for their antioxidative potential. In the tert-butylhydroperoxide (t-BHP)-induced RAW 264.7 macrophage cell line, Desoxyrhapontigenin was the most potent component that reduced intracellular reactive oxygen species (ROS) and peroxynitrite. In response to Desoxyrhapontigenin, the mRNA expression levels of antioxidant enzymes were up-regulated. An electrophoretic mobility shift assay (EMSA) confirmed that Desoxyrhapontigenin promoted the DNA binding of Nrf2 and increased the expression of antioxidant proteins and enzymes regulated by Nrf2. Further investigation utilizing specific inhibitors of Akt, p38, JNK and ERK demonstrated that the phosphatidylinositol 3-kinase (PI3K)/Akt pathway mediates HO-1 expression. Moreover, the increase in Nrf2 expression mediated by treatment with Desoxyrhapontigenin was reversed by Nrf2 or Akt gene knock-down. In the LPS-induced in vivo lung inflammation model, pretreatment with Desoxyrhapontigenin markedly ameliorated LPS-induced lung inflammation and histological changes. Immunohistochemical analysis of Nrf2, HO-1 and p65 was conducted and confirmed that treatment with Desoxyrhapontigenin induced Nrf2 and HO-1 expression but reduced p65 expression. These findings suggest that Desoxyrhapontigenin may be a potential therapeutic candidate as an antioxidant or an anti-inflammatory agent.
Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells.[Pubmed:24295651]
Int Immunopharmacol. 2014 Jan;18(1):182-90.
This study investigates the anti-inflammatory effects of a stilbene compound, Desoxyrhapontigenin, which was isolated from Rheum undulatum. To determine the anti-inflammatory effects of this compound, lipopolysaccharide (LPS)-induced RAW 264.7 macrophages were treated with different concentrations of six stilbene derivatives. The results indicated that compared with other stilbene compounds, Desoxyrhapontigenin (at 10, 30 and 50muM concentrations) significantly inhibited nitric oxide (NO) production, nuclear factor kappa B (NF-kappaB) activation, the protein expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression. Therefore, the anti-inflammatory mechanism of Desoxyrhapontigenin was investigated in detail. The results of this investigation demonstrated that Desoxyrhapontigenin suppressed not only LPS-stimulated pro-inflammatory cytokine secretions, including the secretions of tumor necrosis factor alpha (TNF-alpha) and interleukin-6 (IL-6), but also PGE2 release. As assayed by electrophoretic mobility shift assays (EMSAs), Desoxyrhapontigenin also produced the dose-dependent inhibition of the LPS-induced activation of NF-kappaB and AP-1. Moreover, Desoxyrhapontigenin inhibited the protein expression of myeloid differentiation primary response gene 88 (MyD88), IkappaB kinase (IKK) phosphorylation and the degradation of IkappaBalpha. Activations of p-JNK1 and p-Akt were also significantly inhibited, and phosphorylation of p38 and ERK was down-regulated. A further study revealed that Desoxyrhapontigenin (5 and 25mg/kg, i.p.) reduced paw swelling in carrageenan-induced acute inflammation model in vivo. On the whole, these results indicate that Desoxyrhapontigenin showed anti-inflammatory properties by the inhibition of iNOS and COX-2 expression via the down-regulation of the MAPK signaling pathways and the inhibition of NF-kappaB and Akt activation.
Hypoglycemic and antioxidant effects of Rheum franzenbachii extract in streptozotocin-induced diabetic rats.[Pubmed:20645745]
Pharm Biol. 2010 Jun;48(6):703-7.
The hypoglycemic and antioxidant effects of ethanol extract from the roots and rhizomes of Rheum franzenbachii Munt. (Polygonaceae) were evaluated in streptozotocin-induced diabetic rats. Effects of repeated oral administration of ethanol extract (125, 250, and 500 mg/kg body weight) on the plasma glucose level (PGL), oral glucose tolerance test (OGTT), malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) in diabetic rats were examined. It was found that administration of ethanol extract (125, 250, and 500 mg/kg) produced a significant fall in PGL, AUC, and MDA, while elevating the GSH levels and SOD and CAT activities in diabetic rats. The dose of 500 mg/kg was identified as the most effective dose, with a decrease of 65.8 and 44.0% in PGL and MDA, and elevation of 72.6, 75.0, and 51.5% in GSH level and SOD and CAT activities, respectively, after 14 days of ERF administration in diabetic rats. Moreover, the OGTT studies showed a maximum reduction in PGL and AUC. From the active extract of Rheum franzenbachii, two stilbenes, Desoxyrhapontigenin (1) and desoxyrhaponticin (2), were isolated as major constituents. The present study concludes that the ethanol extract of roots and rhizomes from Rheum franzenbachii had significant hypoglycemic and antioxidant effects.
Inhibition of Monoamine Oxidase by Stilbenes from Rheum palmatum.[Pubmed:28243286]
Iran J Pharm Res. 2016 Fall;15(4):885-892.
Seven stilbenes and one catechin were bioactivity-guidedly isolated from the rhizomes of Rheum palmatem. Their structures were identified as piceatannol (1), resveratrol (2), piceid (3), rhapontigenin (4), piceatannol-3 -O-beta-D-glucopyranoside (5), rhaponticin (6), catechin (7) and Desoxyrhapontigenin (8). Anti-monoamine oxidase (MAO) activities of compounds 1-8 were tested. Compounds 1 and 8 showed significant MAO inhibitory activities with IC50 values 16.4 +/- 1.5 muM and 11.5 +/- 1.1, respectively, when the IC50 value of iproniazid as a standard was 6.5 +/- 0.5 muM. The selectivity of compounds 1-8 against MAO-A and MAO-B were also evaluated. The results showed that compounds 468 preferred to inhibit MAO-A rather than MAO-B with selectivity values ([IC50 of MAO-B]/ [IC50 of MAO-A]) of 4.74, 10.01 and 9.42, respectively. The preliminary structure-activity relationships (SARs) of these compounds were discussed and the molecular modeling was also performed to explore the binding mode of inhibitors at the active site of MAO-A and MAO-B.
Activity-guided isolation of antioxidants from the roots of Rheum emodi.[Pubmed:22568567]
Nat Prod Res. 2013;27(10):946-9.
An activity-guided isolation and purification process was used to identify the DPPH free radical scavenging components of Rheum emodi. The activity-guided isolation revealed that eugenol, gallic acid, quercetin, rutin, epicatechin, Desoxyrhapontigenin, rhapontigenin and mesopsin are the major phenolic compounds responsible for the antioxidant activity of the roots of R. emodi.


