Tripterygium wilfordii
Tripterygium wilfordii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
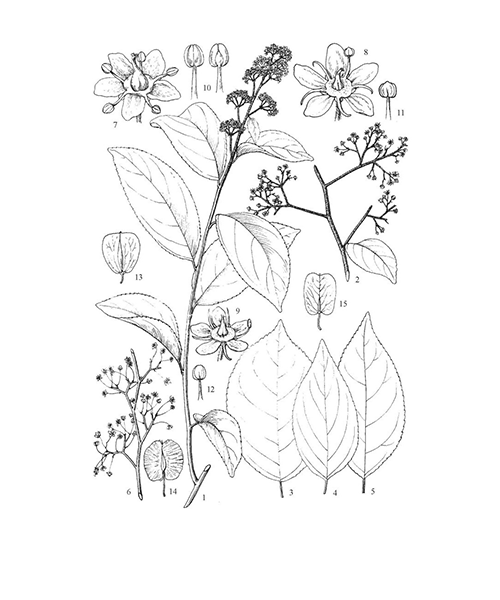
Natural products/compounds from Tripterygium wilfordii
- Cat.No. Product Name CAS Number COA
-
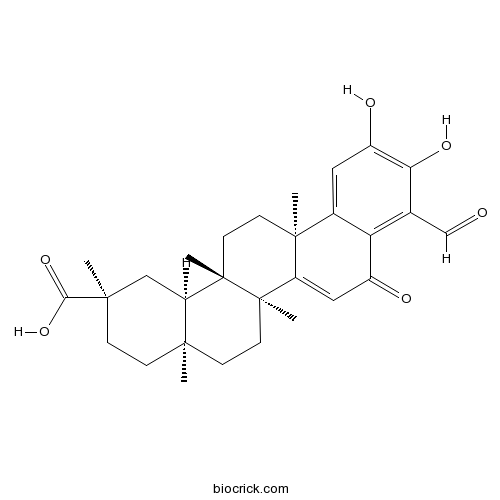
BCN2282
Demethylzeylasteral107316-88-1
Instructions
-
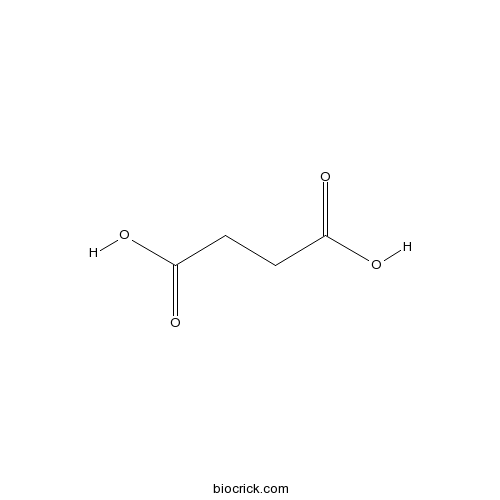
BCN5890
Succinic acid110-15-6
Instructions
-
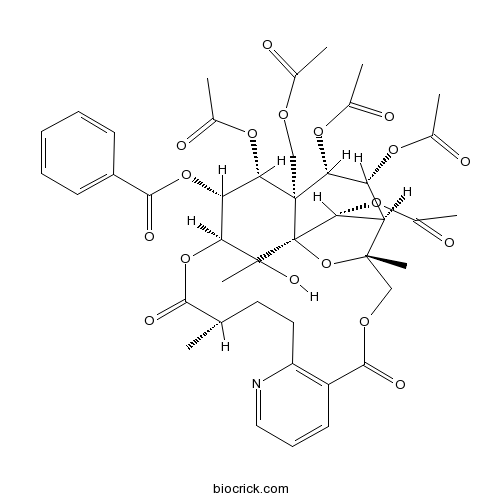
BCN5994
Wilforine11088-09-8
Instructions
-
BCC8980
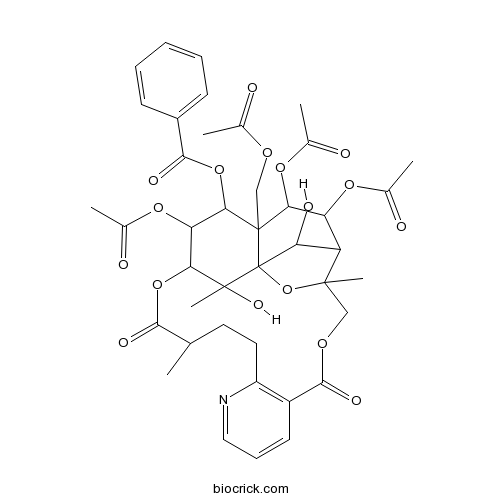
Euojaponine D128397-42-2
Instructions
-
BCN6214
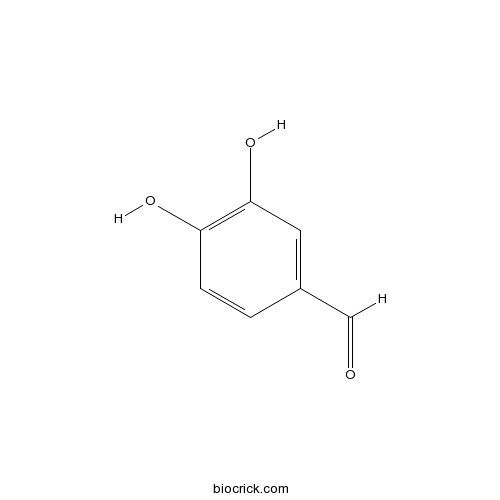
3,4-Dihydroxybenzaldehyde139-85-5
Instructions
-
BCN3095
Triptonine B168009-85-6
Instructions

-
BCC8999
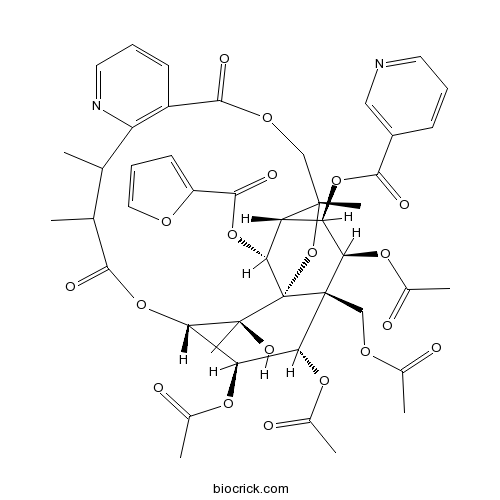
Hyponine E226975-99-1
Instructions
-
BCC8998
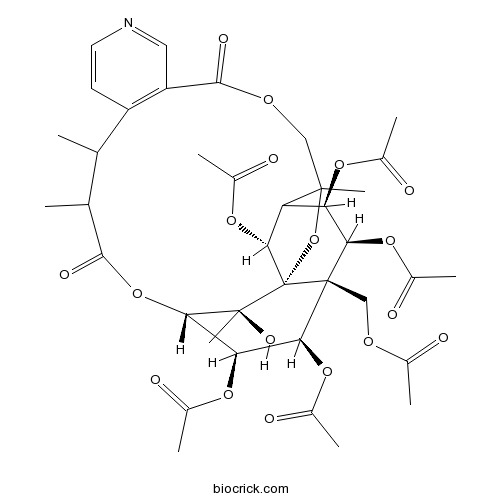
Hyponine D259823-31-9
Instructions

-
BCC9117
Peritassine A262601-67-2
Instructions
-
BCN6315
Procyanidin B229106-49-8
Instructions
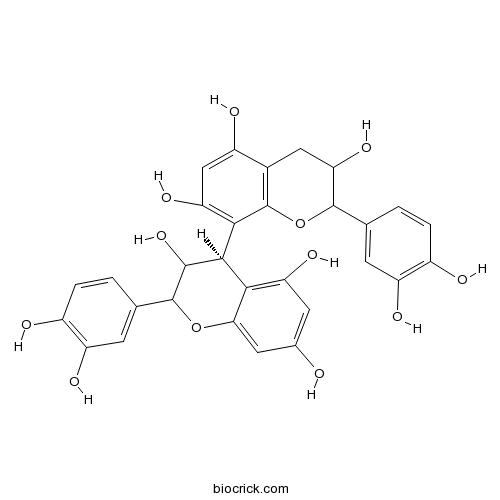
-
BCN3087
Evonine33458-82-1
Instructions
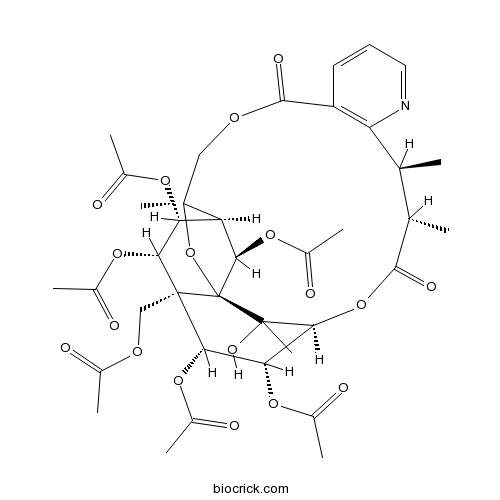
-
BCN5986
Celastrol34157-83-0
Instructions
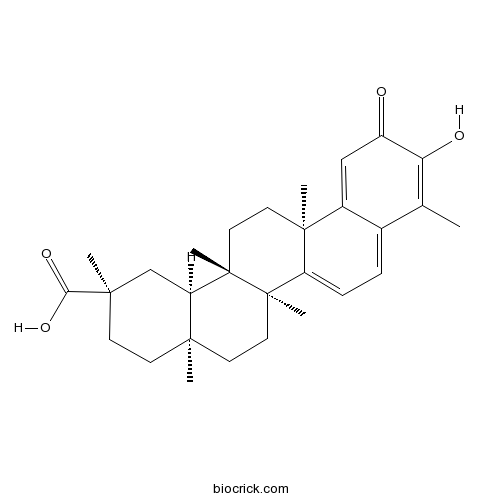
-
BCN3099
Wilfornine A345954-00-9
Instructions
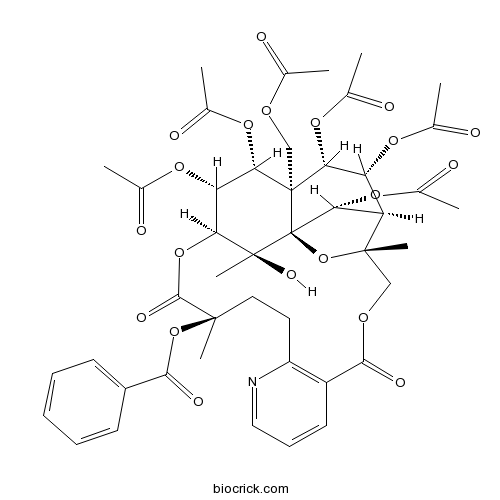
-
BCN5427
Wilforgine37239-47-7
Instructions
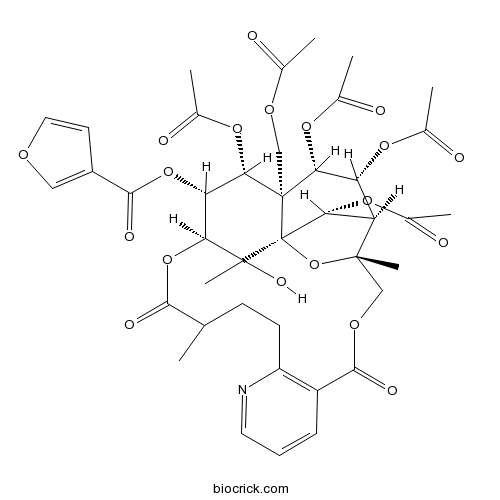
-
BCN3085
Wilfortrine37239-48-8
Instructions
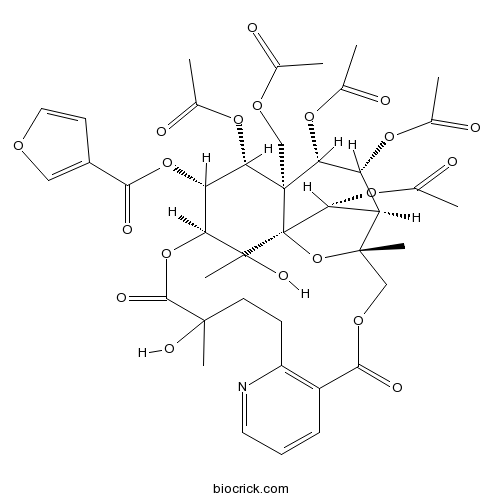
-
BCN3083
Wilfordine37239-51-3
Instructions
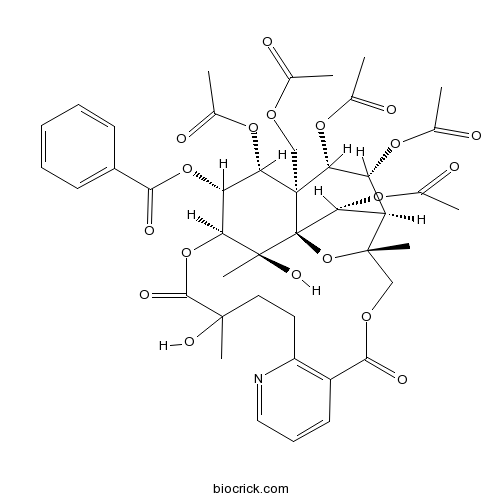
-
BCN5924
Triptonide38647-11-9
Instructions
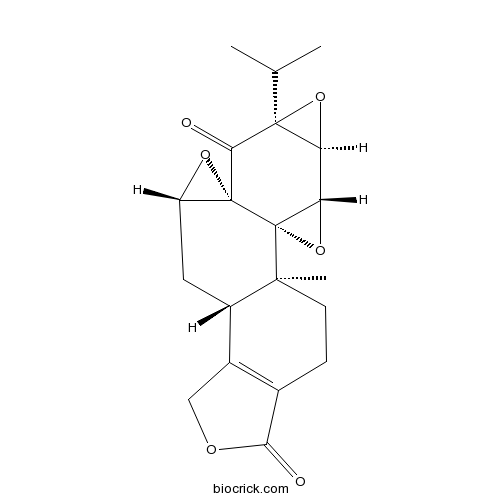
-
BCN5984
Triptolide38748-32-2
Instructions
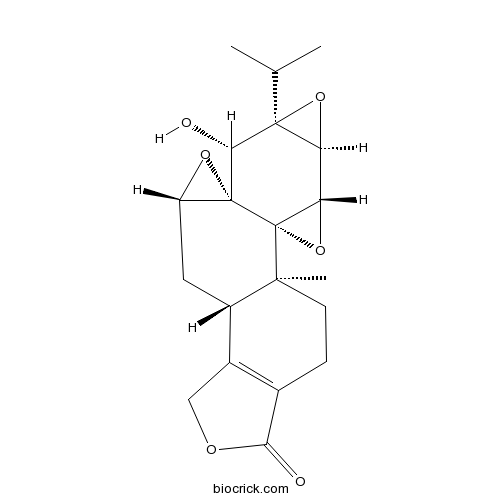
-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN2546
Triptophenolide74285-86-2
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

-
BCN4383
Wilforlide A84104-71-2
Instructions
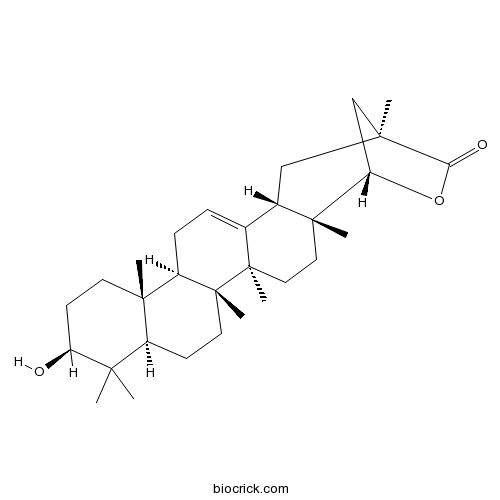
-
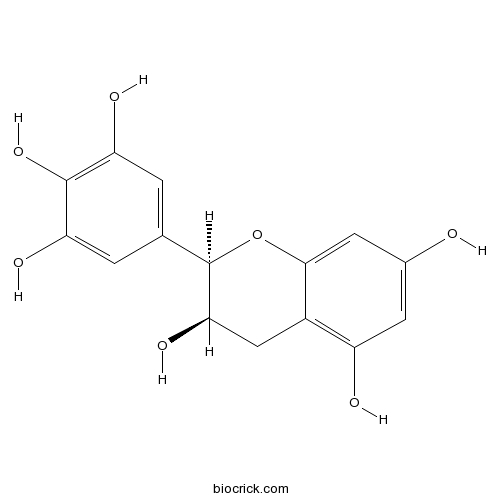
BCN4519
(-)-Epigallocatechin(EGC)970-74-1
Instructions
Preclinical Pharmacokinetics of Triptolide: A Potential Antitumor Drug.[Pubmed: 30112986]
Triptolide, a bioactive component in Tripterygium wilfordii extracts, possess strong anti-proliferative activity on all 60-national cancer institute (NCI) cancer cell lines. The antitumor property of triptolide has made it become a promising anti-cancer drug. However, the widespread use of triptolide in the clinical practice is greatly limited for its multi-organ toxicity and narrow therapeutic window. All the toxic characteristics of triptolide are associated with the pharmacokinetics especially its distribution and accumulation in the target organ. The article presents a comprehensive review of the preclinical pharmacokinetics of triptolide. Oral triptolide is rapidly and highly absorbed. Grapefruit juice affects oral absorption, increasing the area under the concentration-time curve (AUC) by 153 % and the maximum concentration (Cmax) by 141 %. The AUC and the Cmax are not dose proportional. Triptolide distributes into the liver, heart, spleen, lung and kidney. Biotransformation of triptolide in rats includes hydroxylation, sulfate, glucuronide, N-acetylcysteine (NAC) and glutathione (GSH) conjugation and combinations of these pathways. Less than 4 % of triptolide was recovered from the feces, bile and urine within 24 h. After repeating dosage, triptolide was eliminated quickly without accumulation in vivo. As a substrate of P-glycoprotein (P-gp) and CYP3A4, triptolide could have clinically significant pharmacokinetic interactions with those proteins substrates/inhibitors.
[Chemical Constituents of An Endophytic Fungus from Tripterygium wilfordii and Their Monoamine Oxidase Inhibitory Activity}[Pubmed: 30088877]
To study the chemical constituents and monoamine oxidase inhibitory activity of Talaromyces wortmannii,an endophytic fungus was isolated from Tripterygium wilfordii.


