2,16,19-Kauranetriol 2-O-beta-D-allopyranosideCAS# 195723-38-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 195723-38-7 | SDF | Download SDF |
| PubChem ID | 91668399 | Appearance | Powder |
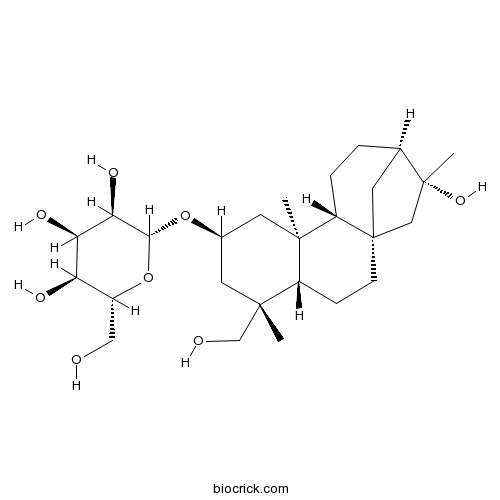
| Formula | C26H44O8 | M.Wt | 484.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4R,5S,6R)-2-[[(1S,4S,5R,7S,9S,10R,13R,14R)-14-hydroxy-5-(hydroxymethyl)-5,9,14-trimethyl-7-tetracyclo[11.2.1.01,10.04,9]hexadecanyl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC1(CC(CC2(C1CCC34C2CCC(C3)C(C4)(C)O)C)OC5C(C(C(C(O5)CO)O)O)O)CO | ||
| Standard InChIKey | QCOICVPTIKZOPU-YCROVFPPSA-N | ||
| Standard InChI | InChI=1S/C26H44O8/c1-23(13-28)9-15(33-22-21(31)20(30)19(29)16(11-27)34-22)10-24(2)17(23)6-7-26-8-14(4-5-18(24)26)25(3,32)12-26/h14-22,27-32H,4-13H2,1-3H3/t14-,15-,16-,17-,18+,19-,20-,21-,22-,23+,24-,25-,26+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,16,19-Kauranetriol 2-O-beta-D-allopyranoside Dilution Calculator

2,16,19-Kauranetriol 2-O-beta-D-allopyranoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0636 mL | 10.3178 mL | 20.6356 mL | 41.2712 mL | 51.5889 mL |
| 5 mM | 0.4127 mL | 2.0636 mL | 4.1271 mL | 8.2542 mL | 10.3178 mL |
| 10 mM | 0.2064 mL | 1.0318 mL | 2.0636 mL | 4.1271 mL | 5.1589 mL |
| 50 mM | 0.0413 mL | 0.2064 mL | 0.4127 mL | 0.8254 mL | 1.0318 mL |
| 100 mM | 0.0206 mL | 0.1032 mL | 0.2064 mL | 0.4127 mL | 0.5159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl 4-O-feruloylquinate
Catalog No.:BCC9041
CAS No.:195723-10-5
- Piromidic Acid
Catalog No.:BCC3840
CAS No.:19562-30-2
- AP1903
Catalog No.:BCC5361
CAS No.:195514-63-7
- Chiirirhamnin
Catalog No.:BCN3179
CAS No.:195450-50-1
- Wortmannin
Catalog No.:BCC3874
CAS No.:19545-26-7
- Bay 11-7821(BAY 11-7082)
Catalog No.:BCC2244
CAS No.:19542-67-7
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Alphitolic acid
Catalog No.:BCN1189
CAS No.:19533-92-7
- Galantamine hydrobromide
Catalog No.:BCN2869
CAS No.:1953-04-4
- Tiopronin (Thiola)
Catalog No.:BCC3870
CAS No.:1953-02-2
- GLP-2 (rat)
Catalog No.:BCC5892
CAS No.:195262-56-7
- N-(3-Methoxybenzyl)oleamide
Catalog No.:BCC6942
CAS No.:883715-21-7
- (+-)-Byakangelicin
Catalog No.:BCN5000
CAS No.:19573-01-4
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
- 2,6,16-Kauranetriol 2-O-beta-D-allopyranoside
Catalog No.:BCN1509
CAS No.:195735-16-1
- HTMT dimaleate
Catalog No.:BCC6736
CAS No.:195867-54-0
- (R)-Nepicastat HCl
Catalog No.:BCC4315
CAS No.:195881-94-8
- 25S-Inokosterone
Catalog No.:BCN3873
CAS No.:19595-18-7
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression.[Pubmed:28280274]
Leukemia. 2017 Nov;31(11):2336-2346.
CXCR4 is a key player in the retention and survival of human acute myeloid leukemia (AML) blasts in the bone marrow (BM) microenvironment. We studied the effects of the CXCR4 antagonist BL-8040 on the survival of AML blasts, and investigated the molecular mechanisms by which CXCR4 signaling inhibition leads to leukemic cell death. Treatment with BL-8040 induced the robust mobilization of AML blasts from the BM. In addition, AML cells exposed to BL-8040 underwent differentiation. Furthermore, BL-8040 induced the apoptosis of AML cells in vitro and in vivo. This apoptosis was mediated by the upregulation of miR-15a/miR-16-1, resulting in downregulation of the target genes BCL-2, MCL-1 and cyclin-D1. Overexpression of miR-15a/miR-16-1 directly induced leukemic cell death. BL-8040-induced apoptosis was also mediated by the inhibition of survival signals via the AKT/ERK pathways. Importantly, treatment with a BCL-2 inhibitor induced apoptosis and act together with BL-8040 to enhance cell death. BL-8040 also synergized with FLT3 inhibitors to induce AML cell death. Importantly, this combined treatment prolonged the survival of tumor-bearing mice and reduced minimal residual disease in vivo. Our results provide a rationale to test combination therapies employing BL-8040 and BCL-2 or FLT3 inhibitors to achieve increased efficacy of these agents.
CXCL-16, IL-17, and bone morphogenetic protein 2 (BMP-2) are associated with overweight and obesity conditions in middle-aged and elderly women.[Pubmed:28293269]
Immun Ageing. 2017 Mar 11;14:6.
BACKGROUND: The current concept of overweight/obesity is most likely related to a combination of increased caloric intake and decreased energy expenditure. Widespread inflammation, associated with both conditions, appears to contribute to the development of some obesity-related comorbidities. Interventions that directly or indirectly target individuals at high risk of developing obesity have been largely proposed because of the increasing number of overweight/obese cases worldwide. The aim of the present study was to assess CXCL16, IL-17, and BMP-2 plasma factors in middle-aged and elderly women and relate them to an overweight or obese status. In total, 117 women were selected and grouped as eutrophic, overweight, and obese, according to anthropometric parameters. Analyses of anthropometric and circulating biochemical parameters were followed by plasma immunoassays for CXCL-16, IL-17, and BMP-2. RESULTS: Plasma mediators increased in all overweight and obese individuals, with the exception of BMP-2 in the elderly group, whereas CXCL16 levels were shown to differentiate overweight and obese individuals. Overweight and/or obese middle-aged and elderly individuals presented with high LDL, triglycerides, and glycemia levels. Anthropometric parameters indicating increased-cardiovascular risk were positively correlated with CXCL-16, BMP-2, and IL-17 levels in overweight and obese middle-aged and elderly individuals. CONCLUSION: This study provides evidence that CXCL-16, IL-17, and BMP-2 are potential plasma indicators of inflammatory status in middle-aged and elderly women; therefore, further investigation of obesity-related comorbidities is recommended. CXCL16, in particular, could be a potential marker for middle-aged and elderly individuals transitioning from eutrophic to overweight body types, which represents an asymptomatic and dangerous condition.
Higher body mass index in 16-19 year-old Jewish Adolescents of North African, Middle Eastern and European Origins is a Predictor of Acute Myeloid Leukemia: a cohort of 2.3 million Israelis.[Pubmed:28258513]
Cancer Causes Control. 2017 Apr;28(4):331-339.
PURPOSE: Studies evaluating adolescent risk factors for developing acute myeloid leukemia (AML) are virtually nonexistent. We assessed adolescent predictors of AML in adults, with a main focus on adolescent BMI. METHODS: The study included 2,310,922 16-19-year-old Jewish Israeli adolescents (mean age 17.3 +/- 0.4, 59.5% male), called up for an obligatory health examination. Sociodemographic and health data, including measured weight and height, were gathered. Body mass index (BMI) was examined both as a continuous variable and grouped according to the World Health Organization (WHO) classification and US-CDC percentiles. Bone-marrow-biopsy-verified AML cases diagnosed up to 31 December 2012 were identified by linkage to the Israel national cancer registry. Multivariable-adjusted Cox proportional-hazards models were used to model time to diagnosis. RESULTS: During 47 million person years of follow-up, 568 AML cases were identified (crude incidence rate 1.21/100,000 person years). There was a multivariable-adjusted hazard ratio (HR) of 1.041 (95% CI 1.015-1.068, p = 0.002) per unit BMI. The association was evident in those of Middle Eastern, North African, and European origin. A graded association was evident across the overweight and obese WHO grouping. With the US-CDC grouping, excess risk was evident in overweight but not in obese adolescents, although a test for trend in percentiles was significant (p = 0.004). Borderline associations were noted for origin (p = 0.065) (higher in the predominantly Ashkenazi European origin), sex (higher in women: HR = 1.24 (95% CI 0.99-1.55), and stature (HR = 1.013, 95% CI 1.000-1.026, per cm). CONCLUSIONS: Higher BMI in adolescence was associated with increased AML incidence in adulthood in this multiethnic population.
Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J-STEP/INS): Results of a 16-week randomized, double-blind, placebo-controlled multicentre trial.[Pubmed:28371205]
Diabetes Obes Metab. 2017 Oct;19(10):1397-1407.
AIMS: To assess the effects of 16 weeks of tofogliflozin (sodium-glucose co-transporter-2 [SGLT2] inhibitor) treatment vs placebo on glycated haemoglobin (HbA1c) levels in Japanese patients with type 2 diabetes mellitus (T2DM) inadequately controlled with insulin monotherapy or insulin plus a dipeptidyl peptidase-4 (DPP-4) inhibitor. METHODS: The study comprised a 16-week, multicentre, double-blind, placebo-controlled period and a 36-week extension (NCT02201004). Men and women (aged >/=20 and /=7.5% and


