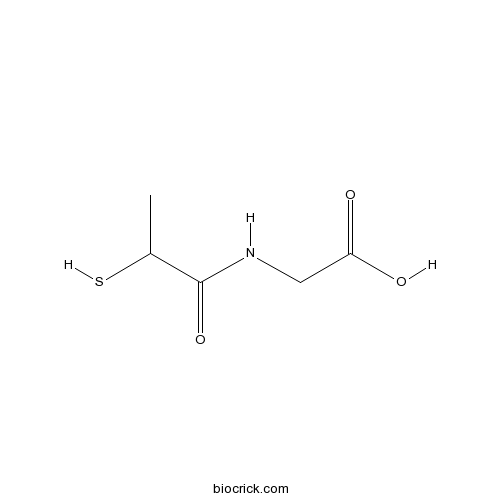
Tiopronin (Thiola)Drug for cystinuria treatment CAS# 1953-02-2 |
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Sotalol
Catalog No.:BCC4356
CAS No.:3930-20-9
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Metoprolol Tartrate
Catalog No.:BCC4330
CAS No.:56392-17-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1953-02-2 | SDF | Download SDF |
| PubChem ID | 5483 | Appearance | Powder |
| Formula | C5H9NO3S | M.Wt | 163.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (612.78 mM) H2O : 100 mg/mL (612.78 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(2-sulfanylpropanoylamino)acetic acid | ||
| SMILES | CC(C(=O)NCC(=O)O)S | ||
| Standard InChIKey | YTGJWQPHMWSCST-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H9NO3S/c1-3(10)5(9)6-2-4(7)8/h3,10H,2H2,1H3,(H,6,9)(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tiopronin is a prescription thiol drug used to control the rate of cystine precipitation and excretion in the disease cystinuria.
Target: Others
Tiopronin is used primarily for cystinuria and is well known in the cystinuric community. Depending on the severity of a person's cystinuria, tiopronin may be taken for life, possibly starting in early childhood. It may also be used for Wilson's disease (an overload of copper in the body), and certain types of rare arthritis, though tiopronin is not an anti-inflammatory. Tiopronin is also sometimes used as a stabilizing agent for metal nanoparticles. The thiol group binds to the nanoparticles, preventing coagulation [1, 2]. References: | |||||

Tiopronin (Thiola) Dilution Calculator

Tiopronin (Thiola) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1278 mL | 30.6391 mL | 61.2783 mL | 122.5565 mL | 153.1957 mL |
| 5 mM | 1.2256 mL | 6.1278 mL | 12.2557 mL | 24.5113 mL | 30.6391 mL |
| 10 mM | 0.6128 mL | 3.0639 mL | 6.1278 mL | 12.2557 mL | 15.3196 mL |
| 50 mM | 0.1226 mL | 0.6128 mL | 1.2256 mL | 2.4511 mL | 3.0639 mL |
| 100 mM | 0.0613 mL | 0.3064 mL | 0.6128 mL | 1.2256 mL | 1.532 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tiopronin (Thiola) is an inhibitor of cystine precipitation and excretion and used for the treatment of cystinuria [1].
Cystinuria is an inherited autosomal recessive disease that is characterized by the formation of cystine stones in the ureter, kidneys and bladder.
In seven cystinuric patients, tiopronin decreased the urinary supersaturation of cystine. The mean cystine capacity without tiopronin was 130.6 + 280.8, while the value during tiopronin use was 43.1 + 131.2 [1]. In 13 patients with cystinuria, the excretion of free cystine increased by 0.75 mg for each mM increase in urinary sodium, which suggested tubular reabsorption of cystine is in a sodium-dependent way [2]. In thirty-one patients with homozygous cystinuria, tiopronin reduced the urinary free cystine excretion by 0.15 mg in a dose-dependant way. Low doses of tiopronin increased urinary excretion of total cystine as well as the mixed disulfide [3]. In 13 patients with cystinuria, tiopronin decreased urinary cystine excretion from 901.48 mg to 488.60 mg [4].
References:
[1]. Dolin DJ, Asplin JR, Flagel L, et al. Effect of cystine-binding thiol drugs on urinary cystine capacity in patients with cystinuria. J Endourol, 2005, 19(3): 429-432.
[2]. Lindell A, Denneberg T, Edholm E, et al. The effect of sodium intake on cystinuria with and without tiopronin treatment. Nephron, 1995, 71(4): 407-415.
[3]. Lindell A, Denneberg T, Jeppsson JO. Urinary excretion of free cystine and the tiopronin-cysteine-mixed disulfide during long term tiopronin treatment of cystinuria. Nephron, 1995, 71(3): 328-342.
[4]. Koide T, Yoshioka T, Miyake O, et al. Long-term study of tiopronin in patients with cystinuria. Hinyokika Kiyo, 2003, 49(2): 115-120.
- GLP-2 (rat)
Catalog No.:BCC5892
CAS No.:195262-56-7
- N-(3-Methoxybenzyl)oleamide
Catalog No.:BCC6942
CAS No.:883715-21-7
- 4-DAMP
Catalog No.:BCC6661
CAS No.:1952-15-4
- Dihydrosenkyunolide C
Catalog No.:BCC8942
CAS No.:195142-72-4
- 4-Hydroxycinnamamide
Catalog No.:BCN1188
CAS No.:194940-15-3
- Daphnetin 7-methyl ether
Catalog No.:BCN2734
CAS No.:19492-03-6
- ACPT-I
Catalog No.:BCC5702
CAS No.:194918-76-8
- Ginsenoside Rs3
Catalog No.:BCN3716
CAS No.:194861-70-6
- Jujuboside D
Catalog No.:BCN4951
CAS No.:194851-84-8
- Dracoflavan C2
Catalog No.:BCN3586
CAS No.:194794-50-8
- Dracoflavan C1
Catalog No.:BCN3587
CAS No.:194794-49-5
- Dracoflavan B2
Catalog No.:BCN3590
CAS No.:194794-47-3
- Galantamine hydrobromide
Catalog No.:BCN2869
CAS No.:1953-04-4
- Alphitolic acid
Catalog No.:BCN1189
CAS No.:19533-92-7
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bay 11-7821(BAY 11-7082)
Catalog No.:BCC2244
CAS No.:19542-67-7
- Wortmannin
Catalog No.:BCC3874
CAS No.:19545-26-7
- Chiirirhamnin
Catalog No.:BCN3179
CAS No.:195450-50-1
- AP1903
Catalog No.:BCC5361
CAS No.:195514-63-7
- Piromidic Acid
Catalog No.:BCC3840
CAS No.:19562-30-2
- Methyl 4-O-feruloylquinate
Catalog No.:BCC9041
CAS No.:195723-10-5
- 2,16,19-Kauranetriol 2-O-beta-D-allopyranoside
Catalog No.:BCN1510
CAS No.:195723-38-7
- (+-)-Byakangelicin
Catalog No.:BCN5000
CAS No.:19573-01-4
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
Influence of Tiopronin on the Metabolism of Alcohol in Healthy Subjects.[Pubmed:28142160]
Drug Res (Stuttg). 2017 Apr;67(4):204-210.
Drug safety- and drug-alcohol interaction studies have mainly been conducted for frequently prescribed drugs with high financial interests. Orphan drugs such as tiopronin (ORPHA25073) are often neglected in terms of clinical research. Tiopronin is a drug that is mainly used for the treatment of cystinuria. In this study, the interaction of tiopronin regarding the metabolism of alcohol (primary objective), and the safety of tiopronin in combination with alcohol was tested in healthy volunteers.In this randomised, double-blind, cross-over study, 13 healthy subjects received 500 mg tiopronin or an identical looking placebo 1 h before the intake of 0.8 g of alcohol per kg of bodyweight. Blood alcohol concentrations were measured over the course of 12 h after consumption. The experiment was repeated 7 days later with the previous placebo group receiving the active drug and vice-versa. Changes in blood alcohol AUC and elimination rate k were analysed using a 2-tailed t-test. Further acetaldehyde concentrations were measured. Additionally, the concentration ability of the subjects was tested and any adverse effects were recorded.There was no significant change in blood alcohol or acetaldehyde concentration. Significant differences in concentration tests refer presumably to learning effects. No serious adverse event occurred. All adverse events were reversible and there was no significant difference in occurrence between drug and placebo group.It was demonstrated that tiopronin does not affect the metabolism of alcohol. Intake of tiopronin in combination with alcohol has no safety implications on healthy subjects.
Action of Bacopa monnieri to antagonize cisplatin-induced emesis in Suncus murinus (house musk shrew).[Pubmed:28363413]
J Pharmacol Sci. 2017 Apr;133(4):232-239.
Bacopa monnieri (BM, family Scrophulariaceae) is used in several traditional systems of medicine for the management of epilepsy, depression, neuropathic pain, sleep disorders and memory deficits. The present study investigated the potential of BM methanol (BM-MetFr) and BM n-butanol fractions (BM-ButFr) to reduce chemotherapy-induced emesis in Suncus murinus (house musk shrew). Cisplatin (30 mg/kg, i.p.) reliably induced retching and/or vomiting over a 2 day period. BM-MetFr (10-40 mg/kg, s.c.) and BM-ButFr (5-20 mg/kg, s.c.) antagonized the retching and/or vomiting response by approximately 59.4% (p < 0.05) and 78.9% (p < 0.05), respectively, while the 5-HT3 receptor antagonist, palonosetron (0.5 mg/kg, s.c.), reduced the response by approximately 71% (p < 0.05). The free radical scavenger/antioxidant, N-(2-mercaptopropionyl)-glycine (30-300 mg/kg, s.c.) reduced the retching and/or vomiting response occurring on day one non-significantly by 44% (p > 0.05). In conclusion, the n-butanol fractions of BM have anti-emetic activity comparable with palonosetron and MPG. BM may be useful alone or in combination with other anti-emetic drugs for the treatment of chemotherapy-induced emesis in man.
Genotype and Phenotype Analysis in Pediatric Patients with Cystinuria.[Pubmed:28049243]
J Korean Med Sci. 2017 Feb;32(2):310-314.
Cystinuria is an inherited disorder characterized by defective renal reabsorption of cystine and dibasic amino acids leading to nephrolithiasis. This study was conducted to analyze the genotypes and phenotypes of pediatric patients with cystinuria. Eight children from Seoul National University Hospital and Asan Medical Center presenting with cystinuria from January 2003 to June 2016 were retrospectively analyzed. Mutational studies were performed by direct sequencing. Two of the 8 were male and 6 were female. The median ages at onset and diagnosis were 1.5 (range, 0.3-13.6) and 2.6 (range, 0.7-16.7) years, respectively. The median followed up was 7.7 (range, 3.4-14.0) years. Mutational analyses were performed in 7 patients and revealed biallelic SLC3A1 mutations (AA genotype) in 4 patients, a single heterozygous SLC3A1 mutation (A- genotype) in 1 patient, biallelic SLC7A9 mutations (BB genotype) in 1 patient, and a single heterozygous SLC7A9 mutation (B- genotype) in 1 patient. Two of the mutations were novel. No genotype-phenotype correlations were observed, except for earlier onset age in patients with non-AA genotypes than in patients with the AA genotype. All patients suffered from recurrent attacks of symptomatic nephrolithiasis, which lead to urologic interventions. At the last follow-up, 3 patients had a mild-to-moderate degree of renal dysfunction. This is the first study of genotypic and phenotypic analyses of patients with cystinuria in Korea.
Retrograde intrarenal surgery in the management of pediatric cystine stones.[Pubmed:28262541]
J Pediatr Urol. 2017 Oct;13(5):487.e1-487.e5.
OBJECTIVE: To investigate the efficacy and safety of retrograde intrarenal surgery (RIRS) in the treatment of pediatric cystine stones. STUDY DESIGN: Data of the pediatric patients who underwent RIRS for kidney stones were retrospectively evaluated. A total of 14 children with cystine stones managed with RIRS were identified. In addition to the patient demographics and stone characteristics, all retrospectively obtained operative data were evaluated and discussed in detail, with an emphasis on the success and complication rates. RESULTS: Mean age of the 14 cases was 10.9 +/- 2.2 years (range: 7-15). Mean stone size was 13.6 +/- 2.4 mm (range: 10-18) (Summary table). Of these stones, four were located in the renal pelvis, three were in the lower, three were in the middle and the remaining four were located in upper calyx. Ureteral access sheath was used in 12 (85.7%) patients. The double-J ureteral stent was placed pre-operatively in one case and was inserted postoperatively in 12 cases. Mean operation time was 38.2 +/- 7.2 min (range: 30-50). Complications were observed in two cases: mild ureteral laceration in the first and fever on the second postoperative day in the second patient. All of the patients were stone free on sonographic evaluation at the 4-week follow-up evaluation. Although potassium citrate treatment was initiated in 11 patients, tiopronin treatment was initiated in four patients for recurrence prophylaxis during long-term follow-up. During a mean follow-up period of 25.7 +/- 5.2 months, stone recurrence was noted in one patient. DISCUSSION: Treatment of patients with cystine stones is challenging, due to high risk of rapid recurrence in the presence of residual fragments. Besides allowing complete stone clearance in all cases in the current series, RIRS is a highly reproducible method that can be safely performed, even in recurrences. The major limitations of the current study were low number of patients and short follow-up period. CONCLUSION: The results clearly indicated that RIRS is a safe treatment modality in the management of pediatric cystine stones.
[Cystinuria caused by a SLC7A9 missense mutation in Siamese-crossbred littermates in Germany].[Pubmed:28585658]
Tierarztl Prax Ausg K Kleintiere Heimtiere. 2017 Aug 11;45(4):265-272.
Cystinuria is caused by defective proximal renal tubular reabsorption of the amino acids cystine, ornithine, lysine, and arginine (COLA). The low solubility of cystine in mildly acidic urine may lead to the formation of urinary cystine crystals and uroliths. Much progress has been made recently in the diagnosis and understanding of cystinuria in companion animals. In cats, cystinuria affects equally both genders independent of neutering status and, despite being rare, already more cystinuria-causing mutations have been detected in cats compared to dogs. In this study a litter of Siamese-crossbred cats in Germany was assessed clinically for cystinuria and screened for mutations known to cause cystinuria in cats. An adult male castrated cat was presented with cystine crystalluria and calculi-related urinary obstruction and treated with perineal urethrostomy, cystotomy, and medical management. This cat and a neutered male littermate without evidence of urinary tract disease were found to be positive for cystine by urinary nitroprusside test, to have increased urinary COLA values and to be homozygous for the p.Val294Glu mutation in the SLC7A9 gene coding for b(0,+)AT subunit of the b(0,+) renal COLA transporter. Another littermate was non-cystinuric and did not carry this mutation. The same SLC7A9 mutation was previously found in a Maine coon, a Sphinx and a medium-haired cat in North America suggesting a common ancestor and likely first widespread SLC7A9 mutation causing cystinuria in cats. Genetic screening for this mutation may offer a simple and precise mean to diagnose other cats for cystinuria and offer specific management.


