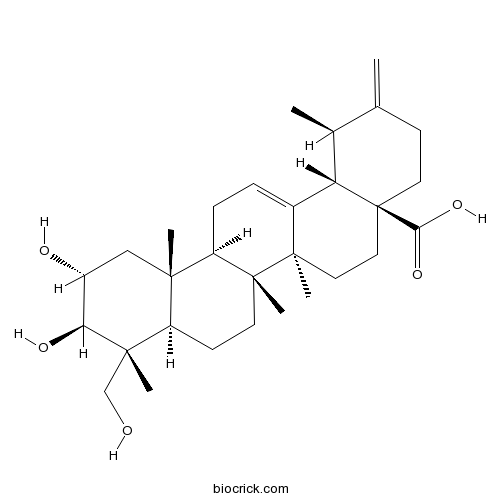
Actinidic acidCAS# 341971-45-7 |
- 3-epi-Actinidic acid
Catalog No.:BCX0427
CAS No.:143839-01-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 341971-45-7 | SDF | Download SDF |
| PubChem ID | 70680338 | Appearance | Powder |
| Formula | C30H46O5 | M.Wt | 486.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4aS,6aR,6aS,6bR,8aR,9R,10R,11R,12aR,14bS)-10,11-dihydroxy-9-(hydroxymethyl)-1,6a,6b,9,12a-pentamethyl-2-methylidene-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1C2C3=CCC4C(C3(CCC2(CCC1=C)C(=O)O)C)(CCC5C4(CC(C(C5(C)CO)O)O)C)C | ||
| Standard InChIKey | FFMVHFPLIIYYNC-QXIFDOFRSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Actinidic acid is a triterpene phytoalexin from unripe kiwi fruit. |

Actinidic acid Dilution Calculator

Actinidic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0547 mL | 10.2733 mL | 20.5465 mL | 41.0931 mL | 51.3663 mL |
| 5 mM | 0.4109 mL | 2.0547 mL | 4.1093 mL | 8.2186 mL | 10.2733 mL |
| 10 mM | 0.2055 mL | 1.0273 mL | 2.0547 mL | 4.1093 mL | 5.1366 mL |
| 50 mM | 0.0411 mL | 0.2055 mL | 0.4109 mL | 0.8219 mL | 1.0273 mL |
| 100 mM | 0.0205 mL | 0.1027 mL | 0.2055 mL | 0.4109 mL | 0.5137 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Propafenone HCl
Catalog No.:BCC5079
CAS No.:34183-22-7
- Pterosin B
Catalog No.:BCN7100
CAS No.:34175-96-7
- H-Tyr-OMe.HCl
Catalog No.:BCC3127
CAS No.:3417-91-2
- Pterosin D
Catalog No.:BCN5269
CAS No.:34169-70-5
- Pterosin Z
Catalog No.:BCN5268
CAS No.:34169-69-2
- Catalponol
Catalog No.:BCN5267
CAS No.:34168-56-4
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Paludosine
Catalog No.:BCN2010
CAS No.:34137-24-1
- (+)-Sophoranol
Catalog No.:BCN3743
CAS No.:3411-37-8
- Sunitinib malate
Catalog No.:BCC3664
CAS No.:341031-54-7
- Nucleozin
Catalog No.:BCC1811
CAS No.:341001-38-5
- Araloside VII
Catalog No.:BCN8129
CAS No.:340982-22-1
- Lys-Bradykinin
Catalog No.:BCC5993
CAS No.:342-10-9
- 3-Methoxyfuran
Catalog No.:BCN5271
CAS No.:3420-57-3
- Dihydroflavokawin B
Catalog No.:BCN5272
CAS No.:3791-76-2
- 7-beta-Hydroxylathyrol
Catalog No.:BCN3413
CAS No.:34208-98-5
- Trachelogenin
Catalog No.:BCN2739
CAS No.:34209-69-3
- Tovopyrifolin C
Catalog No.:BCN6888
CAS No.:34211-53-5
- Echinatin
Catalog No.:BCN6277
CAS No.:34221-41-5
- 3-Acetoxytropane
Catalog No.:BCN1933
CAS No.:3423-26-5
- Tropine acetate
Catalog No.:BCN1922
CAS No.:3423-27-6
- Clozapine N-oxide (CNO)
Catalog No.:BCC1487
CAS No.:34233-69-7
- Telbivudine
Catalog No.:BCC3862
CAS No.:3424-98-4
- Saralasin
Catalog No.:BCC5714
CAS No.:34273-10-4
A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta.[Pubmed:18481026]
Arch Pharm Res. 2008 May;31(5):666-70.
A new coumaroyl triterpene, 3-O-trans-p-coumaroyl Actinidic acid (1), as well as five known triterpenes, ursolic acid (2), 23-hydroxyursolic acid (3), corosolic acid (4), asiatic acid (5) and betulinic acid (6) were isolated from an EtOAc-soluble extract of the roots of Actinidia arguta. The structure of compound 1 was elucidated from interpretation of the spectroscopic data, particularly by extensive 1D and 2D NMR studies. All the isolates (1-6) were evaluated in vitro for their inhibitory activities on pancreatic lipase (PL). Of the isolates, the new compound 1 possessed the highest inhibitory activity on PL, with an IC(50) of 14.95 microM, followed by ursolic acid (2, IC(50) = 15.83 microM). The other four triterpenes (3-6) also showed significant PL inhibitory activity, with IC(50) values ranging from 20.42 to 76.45 microM.
Actinidic acid, a new triterpene phytoalexin from unripe kiwi fruit.[Pubmed:11302196]
Biosci Biotechnol Biochem. 2001 Feb;65(2):480-3.
Seven phytoalexins (1-7), including a new compound, were isolated from the peel of unripe kiwi fruit (Actinidia deliciosa cv. Golden King) that had been wounded and inoculated with Colletotrichum musae. The new phytoalexin (1) was identified as 2alpha,3beta,23-trihydroxy-12,20(30)-ursadien-28-oic acid, and named Actinidic acid. Phytoalexins 2-6 are known triterpenes but have not previously been described as phytoalexins. Phytoalexin 7 is the same triterpene as the phytoalexin of nectarine fruit.


