Tovopyrifolin CCAS# 34211-53-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34211-53-5 | SDF | Download SDF |
| PubChem ID | 5480342 | Appearance | Powder |
| Formula | C14H10O6 | M.Wt | 274.23 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
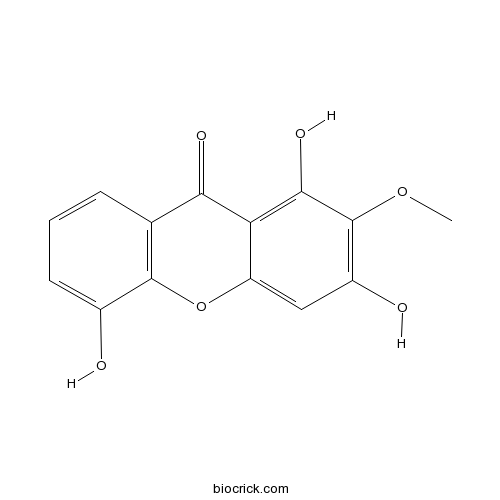
| Chemical Name | 1,3,5-trihydroxy-2-methoxyxanthen-9-one | ||
| SMILES | COC1=C(C=C2C(=C1O)C(=O)C3=C(O2)C(=CC=C3)O)O | ||
| Standard InChIKey | JAOZFCHZESUBKS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O6/c1-19-14-8(16)5-9-10(12(14)18)11(17)6-3-2-4-7(15)13(6)20-9/h2-5,15-16,18H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tovopyrifolin C is a natural product from Calophyllum venulosum. |
| In vitro | Cytotoxicity and structure-activity relationships of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora towards nine human cancer cell lines.[Pubmed: 23381024]Molecules. 2013 Feb 4;18(2):1985-94.
The cytotoxic structure-activity relationships among a series of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora were studied. |
| Structure Identification | J Asian Nat Prod Res. 2015;17(11):1104-8.Venuloxanthone, a new pyranoxanthone from the stem bark of Calophyllum venulosum.[Pubmed: 26023810]
|

Tovopyrifolin C Dilution Calculator

Tovopyrifolin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6466 mL | 18.2329 mL | 36.4657 mL | 72.9315 mL | 91.1644 mL |
| 5 mM | 0.7293 mL | 3.6466 mL | 7.2931 mL | 14.5863 mL | 18.2329 mL |
| 10 mM | 0.3647 mL | 1.8233 mL | 3.6466 mL | 7.2931 mL | 9.1164 mL |
| 50 mM | 0.0729 mL | 0.3647 mL | 0.7293 mL | 1.4586 mL | 1.8233 mL |
| 100 mM | 0.0365 mL | 0.1823 mL | 0.3647 mL | 0.7293 mL | 0.9116 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trachelogenin
Catalog No.:BCN2739
CAS No.:34209-69-3
- 7-beta-Hydroxylathyrol
Catalog No.:BCN3413
CAS No.:34208-98-5
- Dihydroflavokawin B
Catalog No.:BCN5272
CAS No.:3791-76-2
- 3-Methoxyfuran
Catalog No.:BCN5271
CAS No.:3420-57-3
- Lys-Bradykinin
Catalog No.:BCC5993
CAS No.:342-10-9
- Actinidic acid
Catalog No.:BCN5270
CAS No.:341971-45-7
- Propafenone HCl
Catalog No.:BCC5079
CAS No.:34183-22-7
- Pterosin B
Catalog No.:BCN7100
CAS No.:34175-96-7
- H-Tyr-OMe.HCl
Catalog No.:BCC3127
CAS No.:3417-91-2
- Pterosin D
Catalog No.:BCN5269
CAS No.:34169-70-5
- Pterosin Z
Catalog No.:BCN5268
CAS No.:34169-69-2
- Catalponol
Catalog No.:BCN5267
CAS No.:34168-56-4
- Echinatin
Catalog No.:BCN6277
CAS No.:34221-41-5
- 3-Acetoxytropane
Catalog No.:BCN1933
CAS No.:3423-26-5
- Tropine acetate
Catalog No.:BCN1922
CAS No.:3423-27-6
- Clozapine N-oxide (CNO)
Catalog No.:BCC1487
CAS No.:34233-69-7
- Telbivudine
Catalog No.:BCC3862
CAS No.:3424-98-4
- Saralasin
Catalog No.:BCC5714
CAS No.:34273-10-4
- Angiotensin 1/2 (1-9)
Catalog No.:BCC1005
CAS No.:34273-12-6
- Gardneramine
Catalog No.:BCN5273
CAS No.:34274-91-4
- Urotensin II-related peptide
Catalog No.:BCC5884
CAS No.:342878-90-4
- SDM25N hydrochloride
Catalog No.:BCC7054
CAS No.:342884-71-3
- Chikusetsusaponin V methyl ester
Catalog No.:BCN3472
CAS No.:34291-22-0
- 2,3-Dihydrohinokiflavone
Catalog No.:BCN6680
CAS No.:34292-87-0
Cytotoxicity and structure-activity relationships of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora towards nine human cancer cell lines.[Pubmed:23381024]
Molecules. 2013 Feb 4;18(2):1985-94.
The cytotoxic structure-activity relationships among a series of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora were studied. Eleven xanthone derivatives identified as mesuarianone (1), mesuasinone (2), mesuaferrin A (3), mesuaferrin B (4), mesuaferrin C (5), 6-deoxyjacareubin (6), caloxanthone C (7), macluraxanthone (8), 1,5-dihydroxyxanthone (9), Tovopyrifolin C (10) and alpha-mangostin (11) were isolated from the three Mesua species. The human cancer cell lines tested were Raji, SNU-1, K562, LS-174T, SK-MEL-28, IMR-32, HeLa, Hep G2 and NCI-H23. Mesuaferrin A (3), macluraxanthone (8) and alpha-mangostin (11) showed strong cytotoxicities as they possess significant inhibitory effects against all the cell lines. The structure-activity relationship (SAR) study revealed that the diprenyl, dipyrano and prenylated pyrano substituent groups of the xanthone derivatives contributed towards the cytotoxicities.
Venuloxanthone, a new pyranoxanthone from the stem bark of Calophyllum venulosum.[Pubmed:26023810]
J Asian Nat Prod Res. 2015;17(11):1104-8.
A new pyranoxanthone, venuloxanthone (1), was isolated from the stem bark of Calophyllum venulosum, together with three other xanthones, Tovopyrifolin C (2), ananixanthone (3) and caloxanthone I (4), along with two common triterpenes, friedelin (5) and lupeol (6). The structures of these compounds were identified using several spectroscopic analyses which are NMR, GCMS and FTIR experiments.


