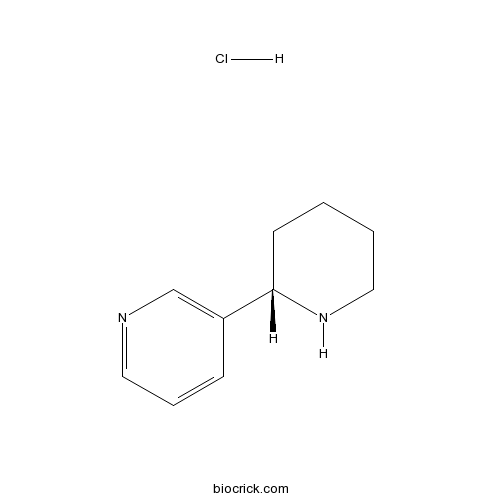
(+)-Anabasine hydrochlorideNeuronal nicotinic receptor partial agonist CAS# 53912-89-3 |
- Olprinone
Catalog No.:BCC1820
CAS No.:106730-54-5
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53912-89-3 | SDF | Download SDF |
| PubChem ID | 3041330 | Appearance | Powder |
| Formula | C10H15ClN2 | M.Wt | 198.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 3-[(2S)-piperidin-2-yl]pyridine;hydrochloride | ||
| SMILES | C1CCNC(C1)C2=CN=CC=C2.Cl | ||
| Standard InChIKey | VTMZQNZVYCJLGG-PPHPATTJSA-N | ||
| Standard InChI | InChI=1S/C10H14N2.ClH/c1-2-7-12-10(5-1)9-4-3-6-11-8-9;/h3-4,6,8,10,12H,1-2,5,7H2;1H/t10-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity neuronal nicotinic ACh receptor partial agonist (Ki values are 0.058, 0.26 and 7.2 μM for rat α7, rat α4β2 and fish skeletal muscle nAChRs respectively). Also stimulates Ca2+-dependent catecholamine release from rat adrenomedullary cells in vitro. |

(+)-Anabasine hydrochloride Dilution Calculator

(+)-Anabasine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.033 mL | 25.1648 mL | 50.3297 mL | 100.6593 mL | 125.8241 mL |
| 5 mM | 1.0066 mL | 5.033 mL | 10.0659 mL | 20.1319 mL | 25.1648 mL |
| 10 mM | 0.5033 mL | 2.5165 mL | 5.033 mL | 10.0659 mL | 12.5824 mL |
| 50 mM | 0.1007 mL | 0.5033 mL | 1.0066 mL | 2.0132 mL | 2.5165 mL |
| 100 mM | 0.0503 mL | 0.2516 mL | 0.5033 mL | 1.0066 mL | 1.2582 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pentostatin
Catalog No.:BCC1845
CAS No.:53910-25-1
- Nb-Feruloyltryptamine
Catalog No.:BCN3899
CAS No.:53905-13-8
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
- Allicin
Catalog No.:BCN2347
CAS No.:539-86-6
- Perillen
Catalog No.:BCN6527
CAS No.:539-52-6
- Hordenine
Catalog No.:BCN1424
CAS No.:539-15-1
- Ticlopidine HCl
Catalog No.:BCC4973
CAS No.:53885-35-1
- Desoxo-narchinol A
Catalog No.:BCN7636
CAS No.:53859-06-6
- Caboxine A
Catalog No.:BCN5715
CAS No.:53851-13-1
- 8-Prenylnaringenin
Catalog No.:BCN2998
CAS No.:53846-50-7
- Flavaprin
Catalog No.:BCN5714
CAS No.:53846-49-4
- 3-(beta-D-Glucopyranosyloxy)-2-hydroxybenzoic acid methyl ester
Catalog No.:BCN7734
CAS No.:53827-68-2
- Hederagenin 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1423
CAS No.:53931-25-2
- Deoxyarbutin
Catalog No.:BCC4774
CAS No.:53936-56-4
- Crotalarine
Catalog No.:BCN2076
CAS No.:53937-97-6
- Euparone
Catalog No.:BCN7204
CAS No.:53947-86-7
- Apterin
Catalog No.:BCN3910
CAS No.:53947-89-0
- Corylin
Catalog No.:BCN5716
CAS No.:53947-92-5
- Aristolactam AII
Catalog No.:BCN3924
CAS No.:53948-07-5
- Aristolactam BII
Catalog No.:BCN5717
CAS No.:53948-09-7
- Aristolactam BIII
Catalog No.:BCN5718
CAS No.:53948-10-0
- Glycyrrhizic acid ammonium salt
Catalog No.:BCN5943
CAS No.:53956-04-0
- Ginsenoside F1
Catalog No.:BCN1244
CAS No.:53963-43-2
- Luteolin 7,3'-di-O-glucuronide
Catalog No.:BCN5396
CAS No.:53965-08-5
[Effect of anabasine hydrochloride on embryogenesis in white rats and rabbits].[Pubmed:7056388]
Farmakol Toksikol. 1982 Jan-Feb;45(1):87-90.
The author reports the results of testing the embryotropic activity of anabasineee hydrochloride in doses of 15, 5, and 3 mg/kg. It was found that oral administration of the drug to pregnant rats in doses exceeding 10-fold (5 mg/kg) and 20-fold (10 mg/kg) the therapeutic ones produced no adverse effect on the fetus. In a dose of 15 mg/kg the drug exerted a moderate embryotoxic action. Multiple oral administration of anabasin hydrochloride to rats in a dose of 3 mg/kg produced no embryotoxic effect. It is concluded that anabasin hydrochloride has no teratogenic properties.
Effect of anabasine on catecholamine secretion from the perfused rat adrenal medulla.[Pubmed:18186309]
J Cardiol. 2007 Dec;50(6):351-62.
OBJECTIVES: The present study was designed to investigate the characteristic effects of anabasine on secretion of catecholamines (CA) from the isolated perfused rat adrenal gland and to establish its mechanism of adrenomedullary secretion. METHODS: The adrenal gland was isolated by a modification of the Wakade method, and perfused with normal Krebs-bicarbonate solution. The content of CA was measured using fluorometry. RESULTS: The perfusion of anabasine(30-300 microM) into an adrenal vein for 60 min resulted in great increases in CA secretions in a dose-dependent fashion. Upon repeated injection of anabasine (100 microM) at 120 min-intervals, CA secretion was rapidly decreased after the third injection of anabasine. However, there was no statistical difference between the CA secretory responses of both 1st and 2nd treated groups by the successive administration of anabasine at 120 min-intervals. Tachyphylaxis to the releasing effects of CA evoked by anabasine was observed by repeated administration. Therefore, in all subsequent experiments, anabasine was not administered successively more than twice at only 120 min-intervals. The CA-releasing effects of anabasine were depressed by pretreatment with chlorisondamine (selective neuronal nicotinic receptor antagonist, 1 microM), atropine (muscarinic receptor antagonist, 2 microM), nicardipine (L-type dihydropyridine Ca2+ channel blocker, 1 microM), TMB-8 (anti-releaser of intracellular Ca2 +, 30 microM), and perfusion of EGTA (Ca2+ chelator, 5 mM) plus Ca2+ -free medium. In the presence of anabasine (100 microM), the CA secretory responses induced by acetylcholine (5.32 mM), high K+ (direct membrane-depolarizer, 56 mM), DMPP(selective neuronal nicotinic receptor agonist, 10(-4) M), and McN-A-343 (selective muscarinic M1 receptor agonist, 10(-4) M) were maximally enhanced in the first 4 min. However, as time elapsed, these responses became more inhibited at later periods. Furthermore, the perfusion of nicotine (30 microM) into an adrenal vein for 60 min also caused a great increase in CA secretion, leading to peak response in the first 0-5 min period. In the presence of nicotine (30 microM), the CA secretory responses induced by acetylcholine, high K+, DMPP and McN-A-343 were also enhanced for the first 4min, but later reduced to less than the control release. CONCLUSIONS: Taken together, these experimental results indicate that anabasine affects rat adrenomedullary CA secretion in a calcium-dependent fashion. This facilitatory effect of anabasine may be mediated by activation of both cholinergic nicotinic and muscarinic receptors, which is relevant to both stimulation of Ca2+ influx into adrenomedullary chromaffin cells and Ca2+ release from cytoplasmic Ca2+ Anabasine may be less potent than nicotine in rat adrenomedullary CA secretion. Anabasine, in addition to nicotine, alkaloids present in tobacco smoke may be a risk factor in causing cardiovascular diseases.
Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists.[Pubmed:10565833]
J Pharmacol Exp Ther. 1999 Dec;291(3):1127-34.
Several neurochemical and electrophysiological studies have shown that neuronal nicotinic receptors are desensitized by pretreatment with lower agonist concentrations than are required to activate the receptors, but the extent of desensitization and agonist concentration required to produce desensitization vary depending upon receptor subtype. Recently, we reported that nicotinic agonists will stimulate the release of [3H]gamma-aminobutyric acid (GABA) from synaptosomes prepared from mouse brain. The studies described herein evaluated desensitization of [3H]GABA release produced by pretreatment with 12 nicotinic agonists. Pretreatment produced near total desensitization that developed slowly (onset T(1/2) = 3.46 min) and was totally reversible (recovery T(1/2) = 4.95 min). Nine of the 12 compounds tested induced total or near total desensitization at concentrations that were less than those required to produce a reliably measured increase in [3H]GABA release. Nicotine produced total block with an IC(50) value of 26 nM. This value is two orders of magnitude lower than the EC(50) for nicotine-induced [3H]GABA release (1630 nM). The three compounds that showed an overlap of the desensitization and activation concentration-effect curves (cytisine, anabasine, nornicotine) are all partial agonists. Comparison of the desensitization properties of the [3H]GABA release with an ion ((86)Rb+) efflux that we have measured previously suggests that the receptor that mediates GABA release and (86)Rb(+) efflux is the same, most likely the alpha4beta2 subtype.
Neuronal nicotinic receptor beta2 and beta4 subunits confer large differences in agonist binding affinity.[Pubmed:9855644]
Mol Pharmacol. 1998 Dec;54(6):1132-9.
We used equilibrium binding analysis to characterize the agonist binding properties of six different rat neuronal nicotinic receptor subunit combinations expressed in Xenopus laevis oocytes. The alpha4beta2 receptor bound [3H]cytisine with a Kdapp of 0.74 +/- 0. 14 nM. The rank order of Kiapp values of additional nicotinic ligands, determined in competition assays, was cytisine < nicotine < acetylcholine < carbachol < curare. These pharmacological properties of alpha4beta2 expressed in oocytes are comparable to published values for the high affinity cytisine binding site in rat brain (alpha4beta2), demonstrating that rat neuronal nicotinic receptors expressed in X. laevis oocytes display appropriate pharmacological properties. Use of [3H]epibatidine allowed detailed characterization of multiple neuronal nicotinic receptor subunit combinations. Kdapp values for [3H]epibatidine binding were 10 pM for alpha2beta2, 87 pM for alpha2beta4, 14 pM for alpha3beta2, 300 pM for alpha3beta4, 30 pM for alpha4beta2, and 85 pM for alpha4beta4. Affinities for six additional agonists (acetylcholine, anabasine, cytisine, 1, 1-dimethyl-4-phenylpiperazinium, lobeline, and nicotine) were determined in competition assays. The beta2-containing receptors had consistently higher affinities for these agonists than did beta4-containing receptors. Particularly striking examples are the affinities displayed by alpha2beta2 and alpha2beta4, which differ in 1,1-dimethyl-4-phenylpiperazinium, nicotine, lobeline, and acetylcholine affinity by 120-, 86-, 85-, and 61-fold, respectively. Although smaller differences in affinity could be ascribed to different alpha subunits, the major factor in determining agonist affinity was the nature of the beta subunit.
Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors.[Pubmed:9399967]
J Pharmacol Exp Ther. 1997 Dec;283(3):979-92.
We assessed the pharmacological activity of anabaseine, a toxin found in certain animal venoms, relative to nicotine and anabasine on a variety of vertebrate nicotinic receptors, using cultured cells, the Xenopus oocyte expression system, contractility assays with skeletal and smooth muscle strips containing nicotinic receptors and in vivo rat prostration assay involving direct injection into the lateral ventricle of the brain. Anabaseine stimulated every subtype of nicotinic receptor that was tested. It was the most potent frog skeletal muscle nicotinic receptor agonist. At higher concentrations it also blocked the BC3H1 (adult mouse) muscle type receptor ion channel. The affinities of the three nicotinoid compounds for rat brain membrane alpha-bungarotoxin binding sites and their potencies for stimulating Xenopus oocyte homomeric alpha7 receptors, expressed in terms of their active monocation concentrations, displayed the same rank order, anabaseine>anabasine> nicotine. Although the maximum currents generated by anabaseine and anabasine at alpha7 receptors were equivalent to that of acetylcholine, the maximum response to nicotine was only about 65% of the acetylcholine response. At alpha4-beta2 receptors the affinities and apparent efficacies of anabaseine and anabasine were much less than that of nicotine. Anabaseine, nicotine and anabasine were nearly equipotent on sympathetic (PC12) receptors, although parasympathetic (myenteric plexus) receptors were much more sensitive to anabaseine and nicotine but less sensitive to anabasine. These differences suggest that there may be different subunit combinations in these two autonomic nicotinic receptors. The preferential interactions of anabaseine, anabasine and nicotine with different receptor subtypes provides molecular clues that should be helpful in the design of selective nicotinic agonists.


