BlumeatinCAS# 118024-26-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118024-26-3 | SDF | Download SDF |
| PubChem ID | 70696494 | Appearance | Powder |
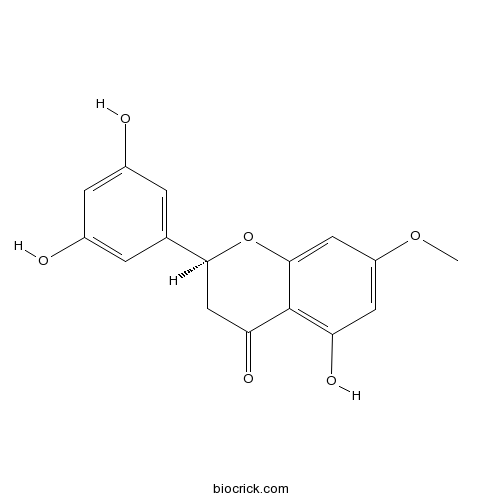
| Formula | C16H14O6 | M.Wt | 302.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(3,5-dihydroxyphenyl)-5-hydroxy-7-methoxy-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC(=C2C(=O)CC(OC2=C1)C3=CC(=CC(=C3)O)O)O | ||
| Standard InChIKey | YEYLMQKEGSQNGZ-AWEZNQCLSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Blumeatin can promote adipocyte differentiation as characterized by increased triglyceride levels in 3T3L1 cells, also can enhance the accumulation of lipid droplets and induced upregulation of the expression of the adipocyte-specific genes aP2 and GLUT4. 2. Blumeatin has antioxidant properties, free radical scavenging activity,and has xanthine oxidase (XO) inhibitory activity. 3. Blumeatin can inhibit the increase of serum alanine aminotransferase (AAT) and liver triglyceride and increased serum triglyceride, beta-lipoprotein, and liver glycogen content in CCl4-intoxicated rats, and can shorten the pentobarbital sleeping time in CCl4-intoxicated mice; suggestes that blumeatin can protect liver against injury induced by CCl4 and TAA. |
| Targets | GLUT |

Blumeatin Dilution Calculator

Blumeatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Acetylepipodophyllotoxin
Catalog No.:BCN6056
CAS No.:1180-35-4
- Ortho-Hydroxyacetophenone
Catalog No.:BCN3827
CAS No.:118-93-4
- Maltol
Catalog No.:BCN4819
CAS No.:118-71-8
- Trimethylgallic acid
Catalog No.:BCN3424
CAS No.:118-41-2
- Syringin
Catalog No.:BCN6059
CAS No.:118-34-3
- Cinchonine
Catalog No.:BCN2464
CAS No.:118-10-5
- Hydrastine
Catalog No.:BCC8187
CAS No.:118-08-1
- Guanosine
Catalog No.:BCN2962
CAS No.:118-00-3
- Rabeprazole sodium
Catalog No.:BCC5227
CAS No.:117976-90-6
- Rabeprazole
Catalog No.:BCC5228
CAS No.:117976-89-3
- Luzindole
Catalog No.:BCC6826
CAS No.:117946-91-5
- PHM 27 (human)
Catalog No.:BCC5869
CAS No.:118025-43-7
- Eriodictyol-6-glucoside
Catalog No.:BCN8026
CAS No.:118040-45-2
- 4-O-Methylgrifolic acid
Catalog No.:BCN7287
CAS No.:118040-60-1
- 7-Hydroxy-3-(4-hydroxybenzyl)chroman
Catalog No.:BCN3578
CAS No.:1180504-64-6
- PS 48
Catalog No.:BCC7859
CAS No.:1180676-32-7
- Dihydroprehelminthosporol
Catalog No.:BCN7288
CAS No.:118069-95-7
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- L-365,260
Catalog No.:BCC7477
CAS No.:118101-09-0
- Prehelminthosporolactone
Catalog No.:BCN7289
CAS No.:118101-72-7
- Cyprodime hydrochloride
Catalog No.:BCC7425
CAS No.:118111-54-9
- Karounidiol
Catalog No.:BCN2704
CAS No.:118117-31-0
- Schisanwilsonin H
Catalog No.:BCN3315
CAS No.:1181216-83-0
Xanthine oxidase inhibitory activities of extracts and flavonoids of the leaves of Blumea balsamifera.[Pubmed:20738223]
Pharm Biol. 2010 Dec;48(12):1405-12.
CONTEXT: Blumea balsamifera DC (Compositae) leaves have been recommended for use as a folk medicine in the treatment of various diseases related to urolithiasis in southeast Asia. Phytochemical studies of this plant revealed it contains four classes of flavonoids (e.g., flavonols, flavones, flavanones, and dihydroflavonol derivatives). OBJECTIVE: In view of the broad pharmacological activity of flavonoids, this study was carried out to determine the xanthine oxidase (XO) inhibitory and enzymatically produced superoxide radical scavenging activity of different organic extracts and that of the isolated flavonoids from B. balsamifera leaves. MATERIALS AND METHODS: The inhibitory activity of XO was assayed spectrophotometrically at 295 nm. The superoxide radicals scavenging activity was assessed by NBT reduction method, spectrophotometrically at 560 nm. A dose response curve was plotted for determining IC(5)(0) values. RESULTS: The methanol extract (IC(5)(0) = 0.111 mg/mL) showed higher XO inhibitory activity than the chloroform (0.138 mg/mL) and pet-ether extracts (0.516 mg/mL). IC(5)(0) values of scavenging of superoxide radicals for extracts decreased in the order of: methanol (0.063 mg/mL) > chloroform (0.092 mg/mL) > pet-ether (0.321 mg/mL). The XO inhibitory activity of the isolated flavonoids and reference compounds tested decreased in the order of: allopurinol > luteolin > quercetin > tamarixetin > 5,7,3',5'-tetrahydroxyflavanone > rhamnetin > luteolin-7-methyl ether > Blumeatin > dihydroquercetin-4'-methyl ether > dihydroquercetin-7,4'-dimethyl ether > L-ascorbic acid. DISCUSSION AND CONCLUSION: The results indicated that the flavone derivatives were more active than the flavonol derivatives. The flavanone derivatives were moderately active and the dihydroflavonol derivatives were the least. The higher flavonoid content of extracts contributed to their higher XO inhibitory activity.
Isoprenylated flavonoid and adipogenesis-promoting constituents of Dodonaea viscosa.[Pubmed:22512738]
J Nat Prod. 2012 Apr 27;75(4):699-706.
Ten new isoprenylated flavonol derivatives, dodoviscins A-J (1-10), and seven known compounds (11-17) were isolated from the aerial parts of Dodonaea viscosa. Compounds 1, 2, 4, 5, 7-9, 5,7,4'-trihydroxy-3',5'-bis(3-methyl-2-buten-1-yl)-3-methoxyflavone (11), 5,7,4'-trihydroxy-3',5'-bis(3-methyl-2-buten-1-yl)-3,6-dimethoxyflavone (12), 5,7,4'-trihydroxy-3'-(4-hydroxy-3-methylbutyl)-5'-(3-methyl-2-buten-1-yl)-3,6-dim ethyoxyflavone (13), sakuranetin (14), and Blumeatin (15) promoted adipocyte differentiation as characterized by increased triglyceride levels in 3T3L1 cells. Compounds 1, 13, and 15 also enhanced the accumulation of lipid droplets and induced upregulation of the expression of the adipocyte-specific genes aP2 and GLUT4.
Simultaneous quantification of flavonoids in blood plasma by a high-performance liquid chromatography method after oral administration of Blumea balsamifera leaf extracts in rats.[Pubmed:23455210]
Pak J Pharm Sci. 2013 Mar;26(2):375-81.
The leaves of Blumea balsamifera are used as a folk medicine in kidney stone diseases in South-East Asia. Phytochemical investigation revealed leaves contained a number of flavonoids. In view of these, the present work was aimed to quantify and preliminary pharmacokinetic investigation of five flavonoids viz. dihydroquercetin-7,4 cent-dimethyl ether (I), dihydroquercetin-4 cent-methyl ether (II), 5,7,3 cent,5 cent-tetrahydroxyflavanone (III), Blumeatin (IV) and quercetin (V) in rat plasma following oral administration (0.5g/Kg) of B. balsamifera leaf extract in rats. Quantification was achieved by using a validated, reproducible high-performance liquid chromatographic method. The mean recoveries of I, II, III, IV and V were 90.6, 93.4, 93.5, 91.2 and 90.3% respectively. The limit of quantification was 25 ng/mL for I and IV, 10 ng/mL for II and III and 100 ng/mL for V respectively. The within day and day-to-day precision for all the compounds were < 10%. The validated HPLC method herein was applied for pharmacokinetic studies and the main pharmacokinetic parameters were: t1/2 (hr) 5.8, 4.3, 2.9, 5.7 and 7.3, Cmax (ng/mL) 594.9, 1542.9 1659.9, 208.9 and 3040.4; Tmax (hr) 4.7, 1.0, 1.0, 3.5 and 2.3; AUC0-oo (ng hr/mL) 5040, 5893, 9260, 1064 and 27233 for I, II, III, IV and V respectively. The developed method was suitable for pharmacokinetic studies and this preliminary study also revealed significant absorption after oral dosing in rats.
[Protective action of blumeatin against experimental liver injuries].[Pubmed:8249641]
Zhongguo Yao Li Xue Bao. 1993 Jul;14(4):376-8.
Blumeatin (Blu, 5,3',5'-trihydroxy-7-methoxy-dihydro-flavone) was first isolated from Blumea balsamifera DC by Department of Chemistry, Sunyatsen University of China. Blu ip inhibited the increase of serum alanine aminotransferase (AAT) and liver triglyceride and increased serum triglyceride, beta-lipoprotein, and liver glycogen content in CCl4-intoxicated rats. Histological lesions of liver were less severe than those of hepatic injury control. Blu ip 0.65 and 3.25 mg.kg-1 inhibited the increase of serum AAT and hepatic TG in thioacetamide (TAA)-intoxicated mice. Blu ip shortened the pentobarbital sleeping time in CCl4-intoxicated mice. It suggested that Blu could protect liver against injury induced by CCl4 and TAA.


