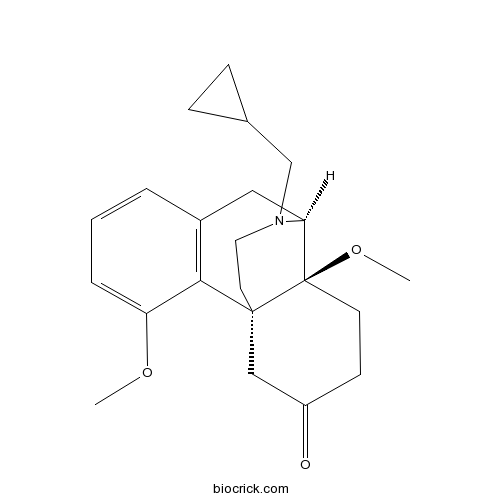
Cyprodime hydrochlorideCAS# 118111-54-9 |
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118111-54-9 | SDF | Download SDF |
| PubChem ID | 5748293 | Appearance | Powder |
| Formula | C22H29NO3 | M.Wt | 355.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| SMILES | COC1=CC=CC2=C1C34CCN(C(C2)C3(CCC(=O)C4)OC)CC5CC5 | ||
| Standard InChIKey | INUCRGMCKDQKNA-CEMLEFRQSA-N | ||
| Standard InChI | InChI=1S/C22H29NO3/c1-25-18-5-3-4-16-12-19-22(26-2)9-8-17(24)13-21(22,20(16)18)10-11-23(19)14-15-6-7-15/h3-5,15,19H,6-14H2,1-2H3/t19-,21-,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective μ-opioid receptor antagonist (Ki values are 5.4, 244.6 and 2187 nM for μ-, δ- and κ-opioid receptors respectively). Reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. |

Cyprodime hydrochloride Dilution Calculator

Cyprodime hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8129 mL | 14.0647 mL | 28.1294 mL | 56.2588 mL | 70.3235 mL |
| 5 mM | 0.5626 mL | 2.8129 mL | 5.6259 mL | 11.2518 mL | 14.0647 mL |
| 10 mM | 0.2813 mL | 1.4065 mL | 2.8129 mL | 5.6259 mL | 7.0323 mL |
| 50 mM | 0.0563 mL | 0.2813 mL | 0.5626 mL | 1.1252 mL | 1.4065 mL |
| 100 mM | 0.0281 mL | 0.1406 mL | 0.2813 mL | 0.5626 mL | 0.7032 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Prehelminthosporolactone
Catalog No.:BCN7289
CAS No.:118101-72-7
- L-365,260
Catalog No.:BCC7477
CAS No.:118101-09-0
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Dihydroprehelminthosporol
Catalog No.:BCN7288
CAS No.:118069-95-7
- PS 48
Catalog No.:BCC7859
CAS No.:1180676-32-7
- 7-Hydroxy-3-(4-hydroxybenzyl)chroman
Catalog No.:BCN3578
CAS No.:1180504-64-6
- 4-O-Methylgrifolic acid
Catalog No.:BCN7287
CAS No.:118040-60-1
- Eriodictyol-6-glucoside
Catalog No.:BCN8026
CAS No.:118040-45-2
- PHM 27 (human)
Catalog No.:BCC5869
CAS No.:118025-43-7
- Blumeatin
Catalog No.:BCN6055
CAS No.:118024-26-3
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Acetylepipodophyllotoxin
Catalog No.:BCN6056
CAS No.:1180-35-4
- Karounidiol
Catalog No.:BCN2704
CAS No.:118117-31-0
- Schisanwilsonin H
Catalog No.:BCN3315
CAS No.:1181216-83-0
- Schisanwilsonin I
Catalog No.:BCN5548
CAS No.:1181216-84-1
- [Phe8Ψ(CH-NH)-Arg9]-Bradykinin
Catalog No.:BCC5995
CAS No.:118122-39-7
- Volvaltrate B
Catalog No.:BCN6736
CAS No.:1181224-13-4
- 6''-O-Acetylastragalin
Catalog No.:BCN6058
CAS No.:118169-27-0
- 6-Oxo-23-norpristimerol
Catalog No.:BCN8054
CAS No.:118172-79-5
- 2-Picenecarboxylic acid
Catalog No.:BCN3063
CAS No.:118172-80-8
- EMD638683
Catalog No.:BCC1551
CAS No.:1181770-72-8
- Soyasaponin Ab
Catalog No.:BCN2896
CAS No.:118194-13-1
- 1,4-Dicaffeoylquinic acid
Catalog No.:BCN5912
CAS No.:1182-34-9
- Isodorsmanin A
Catalog No.:BCN6460
CAS No.:118266-99-2
Involvement of opioid system in behavioral despair induced by social isolation stress in mice.[Pubmed:30551548]
Biomed Pharmacother. 2019 Jan;109:938-944.
Social isolation stress (SIS) as a type of chronic stress could induce depressive- and anxiety-like behaviors. Our study evaluates the role of opioid system on negative behavioral impacts of SIS in male NMRI mice. We investigated effects of morphine, a nonselective opioid receptor (OR) agonist, naltrexone (NLX), an OR antagonist, naltrindole (NLT), a delta opioid receptor (DOR) antagonist, SNC80, a DOR agonist, U-69593, a kappa opioid receptor (KOR) agonist, nor-Binaltorphimine, a selective KOR antagonist and Cyprodime hydrochloride a selective mu opioid receptor (MOR) antagonist on depressive- and anxiety-like behaviors. Using RT-PCR we evaluated ORs gene expression in mice brain. Our findings showed that SIS induced anxiety- and depressive-like behavior in the forced swimming test, open field test, splash test and hole-board test. Moreover, administration of SNC-80 significantly mitigated anxiety- and depressive-like behaviors. NLT decreased grooming-activity in the splash test. Excitingly, administration of agents affecting KOR failed to alter the negative effects of SIS. RT-PCR demonstrated that MOR and KOR gene expression decreased in socially isolated mice; however, SIS did not affect DORs expression. Our findings suggest that SIS at least in part, probably via altering endogenous opioids particularly MORs and KORs but not DORs mediated negative impacts on behavior; also, it could be concluded that DORs might be considered as a novel target for studying depression and anxiety.
Mu- and delta-opioid receptor antagonists reduce levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease.[Pubmed:11520128]
Exp Neurol. 2001 Sep;171(1):139-46.
Long-term treatment of Parkinson's disease with levodopa is complicated by the emergence of involuntary movements, known as levodopa-induced dyskinesia. It has been hypothesized that increased opioid transmission in striatal output pathways may be responsible for the generation of dyskinesia. In this study, we have investigated the effect of blockade of opioid peptide transmission on levodopa-induced dyskinesia in a primate model of Parkinson's disease-the MPTP-lesioned marmoset. Coadministration of nonselective and mu- or delta-subtype-selective opioid receptor antagonists with levodopa resulted in a significant decrease in dyskinesia. There was no attenuation of the anti-parkinsonian actions of levodopa. These data suggest that specific mu- or delta-opioid receptor antagonists might be applicable clinically in the treatment of levodopa-induced dyskinesia in Parkinson's disease.
Mu-opioid receptor specific antagonist cyprodime: characterization by in vitro radioligand and [35S]GTPgammaS binding assays.[Pubmed:10585536]
Eur J Pharmacol. 1999 Oct 27;383(2):209-14.
The use of compounds with high selectivity for each opioid receptor (mu, delta and kappa) is crucial for understanding the mechanisms of opioid actions. Until recently non-peptide mu-opioid receptor selective antagonists were not available. However, N-cyclopropylmethyl-4,14-dimethoxy-morphinan-6-one (cyprodime) has shown a very high selectivity for mu-opioid receptor in in vivo bioassays. This compound also exhibited a higher affinity for mu-opioid receptor than for delta- and kappa-opioid receptors in binding assays in brain membranes, although the degree of selectivity was lower than in in vitro bioassays. Cyprodime has recently been radiolabelled with tritium resulting in high specific radioactivity (36.1 Ci/mmol). We found in in vitro binding experiments that this radioligand bound with high affinity (K(d) 3. 8+/-0.18 nM) to membranes of rat brain affording a B(max) of 87. 1+/-4.83 fmol/mg. Competition studies using mu, delta and kappa tritiated specific ligands confirmed the selective labelling of cyprodime to a mu-opioid receptor population. The mu-opioid receptor selective agonist [D-Ala(2),N-MePhe(4),Gly(5)-ol]enkephalin (DAMGO) was readily displaced by cyprodime (K(i) values in the low nanomolar range) while the competition for delta- ([D-Pen(2), D-Pen(5)]enkephalin (DPDPE)) and kappa- (5alpha,7alpha, 8beta-(-)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro(4, 5)dec-8-yl]-benzene-acetamide (U69,593)) opioid receptor selective compounds was several orders of magnitude less. We also found that cyprodime inhibits morphine-stimulated [35S]GTPgammaS binding. The EC(50) value of morphine increased about 500-fold in the presence of 10 microM cyprodime. These findings clearly indicate that cyprodime is a useful selective antagonist for mu-opioid receptor characterization.
Synthesis and biological evaluation of 14-alkoxymorphinans. 11. 3-Hydroxycyprodime and analogues: opioid antagonist profile in comparison to cyprodime.[Pubmed:7636870]
J Med Chem. 1995 Aug 4;38(16):3071-7.
A series of 3-hydroxy-substituted analogues (3-7) of the mu selective opioid antagonist cyprodime has been synthesized in order to evaluate the role of a hydroxy group at C-3 concerning mu opioid antagonist selectivity. Compounds 3-7 were tested in bioassays (electrical stimulated mouse vas deferens preparation and myenteric-plexus longitudinal muscle preparation of the guinea pig ileum) and opioid receptor binding assays. Antagonism of mu receptor-mediated responses induced by the mu selective agonist DAMGO afforded equilibrium dissociation constants in the mouse vas deferens preparation (Ke values) for compounds 3-7 which agreed closely with their affinities as determined by opioid receptor binding assays (Ki values). At kappa and delta receptors differences were apparent. Although the compounds had high affinity for both kappa and delta receptors in opioid receptor binding, they were very poor at antagonizing agonist responses mediated by kappa and particularly delta agonists in the mouse vas deferens preparation. None of the compounds tested showed agonist potency in the mouse vas deferens preparation or the myenteric-plexus longitudinal muscle preparation of the guinea pig ileum.


