L-365,260CAS# 118101-09-0 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118101-09-0 | SDF | Download SDF |
| PubChem ID | 104929 | Appearance | Powder |
| Formula | C24H22N4O2 | M.Wt | 398.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
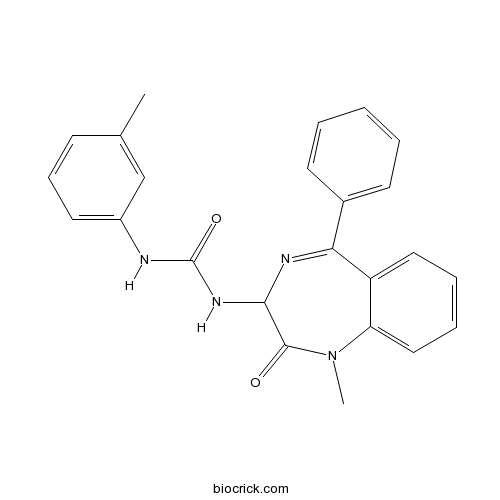
| Chemical Name | 1-(1-methyl-2-oxo-5-phenyl-3H-1,4-benzodiazepin-3-yl)-3-(3-methylphenyl)urea | ||
| SMILES | CC1=CC(=CC=C1)NC(=O)NC2C(=O)N(C3=CC=CC=C3C(=N2)C4=CC=CC=C4)C | ||
| Standard InChIKey | KDFQABSFVYLGPM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective cholecystokinin receptor 2 (CCK2) antagonist (IC50 values are 2 and 280 nM at CCK2 and CCK1 receptors respectively) that is inactive at a range of other receptors including opiate, muscarinic acetylcholine, α- and β adrenergic, histamine, angiotensin and bradykinin receptors. |

L-365,260 Dilution Calculator

L-365,260 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5097 mL | 12.5483 mL | 25.0966 mL | 50.1932 mL | 62.7416 mL |
| 5 mM | 0.5019 mL | 2.5097 mL | 5.0193 mL | 10.0386 mL | 12.5483 mL |
| 10 mM | 0.251 mL | 1.2548 mL | 2.5097 mL | 5.0193 mL | 6.2742 mL |
| 50 mM | 0.0502 mL | 0.251 mL | 0.5019 mL | 1.0039 mL | 1.2548 mL |
| 100 mM | 0.0251 mL | 0.1255 mL | 0.251 mL | 0.5019 mL | 0.6274 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Dihydroprehelminthosporol
Catalog No.:BCN7288
CAS No.:118069-95-7
- PS 48
Catalog No.:BCC7859
CAS No.:1180676-32-7
- 7-Hydroxy-3-(4-hydroxybenzyl)chroman
Catalog No.:BCN3578
CAS No.:1180504-64-6
- 4-O-Methylgrifolic acid
Catalog No.:BCN7287
CAS No.:118040-60-1
- Eriodictyol-6-glucoside
Catalog No.:BCN8026
CAS No.:118040-45-2
- PHM 27 (human)
Catalog No.:BCC5869
CAS No.:118025-43-7
- Blumeatin
Catalog No.:BCN6055
CAS No.:118024-26-3
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Acetylepipodophyllotoxin
Catalog No.:BCN6056
CAS No.:1180-35-4
- Ortho-Hydroxyacetophenone
Catalog No.:BCN3827
CAS No.:118-93-4
- Maltol
Catalog No.:BCN4819
CAS No.:118-71-8
- Prehelminthosporolactone
Catalog No.:BCN7289
CAS No.:118101-72-7
- Cyprodime hydrochloride
Catalog No.:BCC7425
CAS No.:118111-54-9
- Karounidiol
Catalog No.:BCN2704
CAS No.:118117-31-0
- Schisanwilsonin H
Catalog No.:BCN3315
CAS No.:1181216-83-0
- Schisanwilsonin I
Catalog No.:BCN5548
CAS No.:1181216-84-1
- [Phe8Ψ(CH-NH)-Arg9]-Bradykinin
Catalog No.:BCC5995
CAS No.:118122-39-7
- Volvaltrate B
Catalog No.:BCN6736
CAS No.:1181224-13-4
- 6''-O-Acetylastragalin
Catalog No.:BCN6058
CAS No.:118169-27-0
- 6-Oxo-23-norpristimerol
Catalog No.:BCN8054
CAS No.:118172-79-5
- 2-Picenecarboxylic acid
Catalog No.:BCN3063
CAS No.:118172-80-8
- EMD638683
Catalog No.:BCC1551
CAS No.:1181770-72-8
- Soyasaponin Ab
Catalog No.:BCN2896
CAS No.:118194-13-1
L-365,260 reversed effect of sincalide against morphine on electrical and mechanical activities of rat duodenum in vitro.[Pubmed:12100748]
Acta Pharmacol Sin. 2002 Jul;23(7):582-6.
AIM: To study the antagonism of sincalide (CCK-8) to the effect of morphine and its mechanism. METHODS: The electrical and mechanical activities of rat duodenum in vitro were recorded simultaneously. RESULTS: Acetylcholine (ACh, 300 nmol/L) increased the spike potential amplitude (SPA) and the number (SPN) of rat duodenum in vitro, followed by an increase of duodenal contraction amplitudes (CA). The SPA, SPN, and CA of duodenum in vitro were not obviously affected by injection of morphine (330 nmol/L), but it could selectively inhibit the potentiation of ACh. After administration of CCK-8 (0.7 nmol/L), the SPA, SPN, and CA of duodenal segment did not exhibit obvious changes. But CCK-8 could selectively antagonize the effects of morphine, ie, the SPA and SPN were increased again, followed by an increase of CA. CCK-B receptor antagonist L-365,260 (30 nmol/L) reversed the antagonism of CCK-8 to the effect of morphine. CONCLUSION: CCK-8 could selectively antagonize the effect of morphine which inhibited the potentiation of ACh on duodenal activities in vitro. The antagonistic effect of CCK-8 on morphine was mainly mediated by CCK-B receptor.
A phase 1 study of the cholecystokinin (CCK) B antagonist L-365,260 in human subjects taking morphine for intractable non-cancer pain.[Pubmed:12399016]
Neurosci Lett. 2002 Nov 8;332(3):210-2.
To investigate the safety and tolerability of L-365,260 in human subjects taking morphine for intractable pain. An open label study of nine adult subjects. Two doses of L-365,260 were administered to all subjects separated by a 4 h interval (three received 10 mg, three 30 mg and three 60 mg). Haemodynamic and respiratory variables were recorded from immediately prior to first drug administration to T + 600 min. In addition, continuous electrocardiogram (ECG) monitoring and serial 12 lead ECGs were recorded along with pain and side effect measurements. No major side effects were observed. L-365,260 was well tolerated. No abnormalities in blood pressure, heart rate, respiratory rate or ECG measurements were recorded. Minor side effects were observed. L-365,260 can be safely administered at the doses investigated to human subjects receiving morphine for intractable pain.
A randomised, double blind, placebo controlled crossover study of the cholecystokinin 2 antagonist L-365,260 as an adjunct to strong opioids in chronic human neuropathic pain.[Pubmed:12566175]
Neurosci Lett. 2003 Feb 27;338(2):151-4.
The aim of this study was to establish if the cholecystokinin (CCK) 2 antagonist L-365,260 augments the analgesic effect of morphine in human subjects with chronic neuropathic pain. This is a randomised, double blind, placebo controlled study of 40 adult subjects taking morphine for neuropathic pain. Each received placebo, L-365,260 30 mg and L-365,260 120 mg in three divided doses daily separated by a washout period in random order. Pain, activity, sedation, sleep and side effects were recorded along with 12 lead ECGs, renal and liver function tests and full blood pictures. L-365,260 failed to augment the analgesic effect of morphine at any of the dose levels used. Side effects were minor. There were no changes in ECGs and biochemical indices were unaltered with its use. The CCK 2 antagonist L-365,260 does not augment the analgesic effect of morphine in subjects with chronic neuropathic pain. L-365,260 was well tolerated and side effects from its use were minor.
L-365,260 inhibits in vitro acid secretion by interacting with a PKA pathway.[Pubmed:10369481]
Br J Pharmacol. 1999 May;127(1):259-67.
The aim of this study was to analyse the antisecretory mechanism of L-365,260 in vitro in isolated rabbit gastric glands. We showed that compound L-365,260, described as a non-peptide specific competitive CCK-B receptor antagonist, was able to dose-dependently inhibit [14C]-aminopyrine accumulation induced by histamine (10(-4) M), carbachol (5x10(-5) M), 3-isobutyl-1-methyl-xanthine (IBMX) (5x10(-6) M) and forskolin (5x10(-7) M) with similar IC50 values respectively of 1.1+/-0.6x10(-7) M, 1.9+/-1.2x10(-7) M, 4.2+/-2.0x10(-7) M and 4.0+/-2.8x10(-7) M. We showed that L-365,260 acted beyond receptor activation and production of intracellular second messengers and that it had no action on the H+/K+ -ATPase. We found that L-365,260 inhibited cyclic AMP-induced [14C]-aminopyrine accumulation in digitonin-permeabilized rabbit gastric glands, suggesting that this compound acted, at least in part, as an inhibitor of the cyclic AMP-dependent protein kinase (PKA) pathway.
Cholecystokinin action on layer 6b neurons in somatosensory cortex.[Pubmed:19497313]
Brain Res. 2009 Jul 28;1282:10-9.
Layer 6b in neocortex is a distinct sublamina at the ventral portion of layer 6. Corticothalamic projections arise from 6b neurons, but few studies have examined the functional properties of these cells. In the present study we examined the actions of cholecystokinin (CCK) on layer 6b neocortical neurons using whole-cell patch clamp recording techniques. We found that the general CCK receptor agonist CCK8S (sulfated CCK octapeptide) strongly depolarized the neurons, and this action persisted in the presence of tetrodotoxin, suggesting a postsynaptic site of action. The excitatory actions of CCK8S were mimicked by the selective CCK(B) receptor agonist CCK4, and attenuated by the selective CCK(B) receptor antagonist L365260, indicating a role for CCK(B) receptors. Voltage-clamp recordings revealed that CCK8S produced a slow inward current associated with a decreased conductance with a reversal potential near the K(+) equilibrium potential. In addition, intracellular cesium also blocked the inward current, suggesting the involvement of a K(+) conductance, likely K(leak). Our data indicate that CCK, acting via CCK(B) receptors, produces a long-lasting excitation of layer 6b neocortical neurons, and this action may play a critical role in modulation of corticothalamic circuit activity.
A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260.[Pubmed:2721567]
Eur J Pharmacol. 1989 Mar 21;162(2):273-80.
L-365,260 (3R(+)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4- benzodiazepin-3-yl)-N'-(3-methylphenyl)urea), interacted in a stereoselective and competitive manner with guinea pig stomach gastrin and brain cholecystokinin (CCK) receptors. The affinity of L-365,260 for both gastrin (Ki = 1.9 nM) and brain CCK-B (Ki = 2.0 nM) receptors was greater than 2 orders of magnitude higher than its affinity for peripheral pancreatic CCK-A receptors or various other receptors. L-365,260 exhibited a similar high affinity for brain CCK-B receptors of rats, mice and man. A somewhat lower affinity for gastrin and brain CCK-B (IC50 = 20-40 nM) receptors was observed in dog tissues. In vivo, oral administration of L-365,260 antagonized gastrin-stimulated acid secretion in mice (ED50 = 0.03 mg/kg), rats (ED50 = 0.9 mg/kg) and guinea pigs (ED50 = 5.1 mg/kg). L-365,260 did not affect basal acid secretion and did not antagonize histamine- or carbachol-stimulated acid secretion in mice. L-365,260 represents a potentially powerful new tool for investigating gastrin and brain CCK-B receptors, and possibly their role in physiology and disease.


