Rabeprazole sodiumCAS# 117976-90-6 |
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- BAY 61-3606 dihydrochloride
Catalog No.:BCC1407
CAS No.:648903-57-5
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- R406 (free base)
Catalog No.:BCC2553
CAS No.:841290-80-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117976-90-6 | SDF | Download SDF |
| PubChem ID | 14720269 | Appearance | Powder |
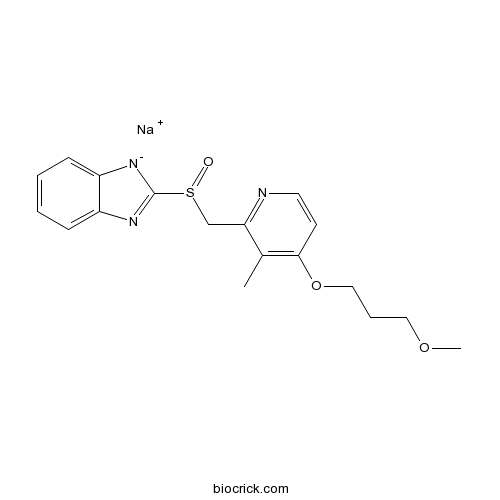
| Formula | C18H20N3NaO3S | M.Wt | 381.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY307640 sodium | ||
| Solubility | DMSO : ≥ 48 mg/mL (125.51 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | sodium;2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl]benzimidazol-1-ide | ||
| SMILES | CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3[N-]2)OCCCOC.[Na+] | ||
| Standard InChIKey | KRCQSTCYZUOBHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H20N3O3S.Na/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18;/h3-4,6-9H,5,10-12H2,1-2H3;/q-1;+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rabeprazole sodium Dilution Calculator

Rabeprazole sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6218 mL | 13.1089 mL | 26.2178 mL | 52.4356 mL | 65.5445 mL |
| 5 mM | 0.5244 mL | 2.6218 mL | 5.2436 mL | 10.4871 mL | 13.1089 mL |
| 10 mM | 0.2622 mL | 1.3109 mL | 2.6218 mL | 5.2436 mL | 6.5545 mL |
| 50 mM | 0.0524 mL | 0.2622 mL | 0.5244 mL | 1.0487 mL | 1.3109 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2622 mL | 0.5244 mL | 0.6554 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Rabeprazole sodium(LY307640 sodium) is an antiulcer drug in the class of proton pump inhibitors. Target: Proton Pump Rabeprazole belongs to a class of antisecretory compounds (substituted benzimidazole proton-pump inhibitors) that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H+/K+ATPase (hydrogen-potassium adenosine triphosphatase) at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, rabeprazole has been characterized as a gastric proton-pump inhibitor. Rabeprazole blocks the final step of gastric acid secretion. In gastric parietal cells, rabeprazole is protonated, accumulates, and is transformed to an active sulfenamide. When studied in vitro, rabeprazole is chemically activated at pH 1.2 with a half-life of 78 seconds.
References:
[1]. http://www.drugbank.ca/drugs/DB01129
- Rabeprazole
Catalog No.:BCC5228
CAS No.:117976-89-3
- Luzindole
Catalog No.:BCC6826
CAS No.:117946-91-5
- GLYX 13
Catalog No.:BCC6013
CAS No.:117928-94-6
- Boc-N-Me-Nle-OH
Catalog No.:BCC2611
CAS No.:117903-25-0
- Forsythoside H
Catalog No.:BCN6431
CAS No.:1178974-85-0
- 7,4'-Dihydroxyhomoisoflavanone
Catalog No.:BCN3582
CAS No.:1178893-64-5
- Fmoc-Thr(Bzl)-OH
Catalog No.:BCC3550
CAS No.:117872-75-0
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
- L-CCG-l
Catalog No.:BCC6609
CAS No.:117857-93-9
- Loreclezole hydrochloride
Catalog No.:BCC7009
CAS No.:117857-45-1
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- Guanosine
Catalog No.:BCN2962
CAS No.:118-00-3
- Hydrastine
Catalog No.:BCC8187
CAS No.:118-08-1
- Cinchonine
Catalog No.:BCN2464
CAS No.:118-10-5
- Syringin
Catalog No.:BCN6059
CAS No.:118-34-3
- Trimethylgallic acid
Catalog No.:BCN3424
CAS No.:118-41-2
- Maltol
Catalog No.:BCN4819
CAS No.:118-71-8
- Ortho-Hydroxyacetophenone
Catalog No.:BCN3827
CAS No.:118-93-4
- Acetylepipodophyllotoxin
Catalog No.:BCN6056
CAS No.:1180-35-4
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Blumeatin
Catalog No.:BCN6055
CAS No.:118024-26-3
- PHM 27 (human)
Catalog No.:BCC5869
CAS No.:118025-43-7
- Eriodictyol-6-glucoside
Catalog No.:BCN8026
CAS No.:118040-45-2
Development and validation of dissolution testings in acidic media for rabeprazole sodium delayed-release capsules.[Pubmed:27066697]
Drug Dev Ind Pharm. 2016 Oct;42(10):1669-77.
Rabeprazole sodium (RAB) dissolved in acidic media is accompanied by its degradation in the course of dissolution testing. To develop and establish the accumulative release profiles of ACIPHEX((R)) Sprinkle (RAB) delayed-release capsules (ACIPHEX((R)) Sprinkle) in acidic media using USP apparatus 2 (paddle apparatus) as a dissolution tester, the issues of determination of accumulative release amount of RAB in these acidic media and interference of hydroxypropylmethyl cellulose phthalate were solved by adding appropriate hydrochloric acid (HCl) into dissolution samples coupled with centrifugation so as to remove the interference and form a solution of degradation products of RAB, which is of a considerably stable ultraviolet (UV) absorbance at the wavelength of 298 nm within 2.0 h. Therefore, the accumulative release amount of RAB in dissolution samples at each sample time points could be determined by UV-spectrophotometry, and the accumulative release profiles of ACIPHEX((R)) Sprinkle in the media of pH 1.0, pH 6.0, and pH 6.8 could be established. The method was validated per as the ICH Q2 (R1) guidelines and demonstrated to be adequate for quality control of ACIPHEX((R)) Sprinkle and the accumulative release profiles can be used as a tool to guide the formulation development and quality control of a generic drug for ACIPHEX((R)) Sprinkle.
Development and validation of HPLC-DAD method for the simultaneous determination of amoxicillin, metronidazole and rabeprazole sodium. Application to spiked simulated intestinal fluid samples.[Pubmed:26024556]
Ann Pharm Fr. 2015 Sep;73(5):351-60.
This work deals with the development, validation and application of an HPLC-DAD method for the determination of a ternary mixture containing amoxicillin (AX), metronidazole (MZ) and the proton pump inhibitor Rabeprazole sodium (RB). This triple therapy is used for treatment of Helicobacter pylori infection. Effective chromatographic separation between the three drugs was achieved using Thermo Hypersil BDS-C8 (4.6x250mm, 5mum particle size) column and a mobile phase composed of phosphate buffer pH 7 and acetonitrile (70: 30, by volume). The mobile phase was pumped isocratically at a flow rate of 1 mL/min. Quantification of the analytes was based on measuring their peak areas at 230nm for both AX and RB, and at 319nm for MZ. AX, MZ and RB eluted at retention times 2.36, 3.55 and 8.72min respectively. The reliability and analytical performance of the proposed HPLC procedure were statistically validated with respect to linearity, ranges, precision, accuracy, selectivity, robustness, detection and quantification limits. The linear dynamic ranges were 25-250, 25-250 and 5-50mug/mL for AX, MZ and RB respectively with correlation coefficients>0.9998. The validated method was successfully applied to the analysis of several laboratory-prepared mixtures as well as simulated intestinal fluid samples spiked with the three drugs.
A study of selective spectrophotometric methods for simultaneous determination of Itopride hydrochloride and Rabeprazole sodium binary mixture: Resolving sever overlapping spectra.[Pubmed:25456673]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt C:1308-15.
Itopride hydrochloride (IT) and Rabeprazole sodium (RB) are co-formulated together for the treatment of gastro-esophageal reflux disease. Three simple, specific and accurate spectrophotometric methods were applied and validated for simultaneous determination of Itopride hydrochloride (IT) and Rabeprazole sodium (RB) namely; constant center (CC), ratio difference (RD) and mean centering of ratio spectra (MCR) spectrophotometric methods. Linear correlations were obtained in range of 10-110mug/muL for Itopride hydrochloride and 4-44mug/mL for Rabeprazole sodium. No preliminary separation steps were required prior the analysis of the two drugs using the proposed methods. Specificity was investigated by analyzing the synthetic mixtures containing the two cited drugs and their capsules dosage form. The obtained results were statistically compared with those obtained by the reported method, no significant difference was obtained with respect to accuracy and precision. The three methods were validated in accordance with ICH guidelines and can be used for quality control laboratories for IT and RB.
Gastrointestinal bleed induced by a fixed dose combination of rabeprazole and diclofenac sodium.[Pubmed:25298591]
Indian J Pharmacol. 2014 Sep-Oct;46(5):555-6.
Non-steroidal anti-inflammatory drugs (NSAIDs) are known to cause gastrointestinal (GI) bleed. Co-administration of proton pump inhibitors (PPIs) has been widely suggested as one of the strategies to prevent these GI complications among NSAIDs users. Herein, we present a case of severe GI bleeding in a patient taking fixed dose combination (FDC) of rabeprazole (20 mg) and diclofenac sodium (100 SR).


