Loreclezole hydrochlorideSubtype-selective GABAA receptor modulator CAS# 117857-45-1 |
- Mosapride
Catalog No.:BCC4078
CAS No.:112885-41-3
- Pizotifen
Catalog No.:BCC4215
CAS No.:15574-96-6
- Pizotifen Malate
Catalog No.:BCC4825
CAS No.:5189-11-7
- Fluvoxamine
Catalog No.:BCC4214
CAS No.:54739-18-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117857-45-1 | SDF | Download SDF |
| PubChem ID | 3034012 | Appearance | Powder |
| Formula | C10H6Cl3N3 | M.Wt | 274.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 110 mg/mL (400.68 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
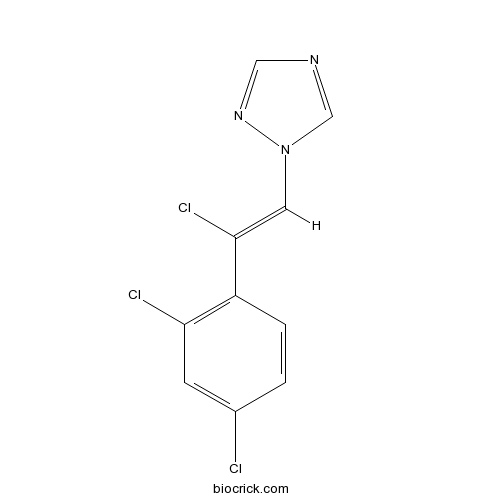
| Chemical Name | 1-[(Z)-2-chloro-2-(2,4-dichlorophenyl)ethenyl]-1,2,4-triazole | ||
| SMILES | C1=CC(=C(C=C1Cl)Cl)C(=CN2C=NC=N2)Cl | ||
| Standard InChIKey | XGLHZTBDUXXHOM-WMZJFQQLSA-N | ||
| Standard InChI | InChI=1S/C10H6Cl3N3/c11-7-1-2-8(9(12)3-7)10(13)4-16-6-14-5-15-16/h1-6H/b10-4- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Subtype-selective GABAA receptor modulator. Acts as a positive allosteric modulator of β2 or β3-subunit containing receptors. Also acts as a negative modulator at a novel regulatory site, enhancing GABAA receptor sensitization. Inhibits homomeric ρ1 GABAC receptors. |

Loreclezole hydrochloride Dilution Calculator

Loreclezole hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.643 mL | 18.2149 mL | 36.4299 mL | 72.8597 mL | 91.0747 mL |
| 5 mM | 0.7286 mL | 3.643 mL | 7.286 mL | 14.5719 mL | 18.2149 mL |
| 10 mM | 0.3643 mL | 1.8215 mL | 3.643 mL | 7.286 mL | 9.1075 mL |
| 50 mM | 0.0729 mL | 0.3643 mL | 0.7286 mL | 1.4572 mL | 1.8215 mL |
| 100 mM | 0.0364 mL | 0.1821 mL | 0.3643 mL | 0.7286 mL | 0.9107 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- 3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No.:BCN8203
CAS No.:1178-24-1
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- AP-III-a4
Catalog No.:BCC5292
CAS No.:1177827-73-4
- Desmethyl-YM 298198
Catalog No.:BCC7365
CAS No.:1177767-57-5
- Decumbenine C
Catalog No.:BCC8314
CAS No.:117772-89-1
- Azithromycin Dihydrate
Catalog No.:BCC4631
CAS No.:117772-70-0
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- SMANT hydrochloride
Catalog No.:BCC6254
CAS No.:1177600-74-6
- N20C hydrochloride
Catalog No.:BCC7292
CAS No.:1177583-87-7
- Forsythoside I
Catalog No.:BCN6430
CAS No.:1177581-50-8
- L-CCG-l
Catalog No.:BCC6609
CAS No.:117857-93-9
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
- Fmoc-Thr(Bzl)-OH
Catalog No.:BCC3550
CAS No.:117872-75-0
- 7,4'-Dihydroxyhomoisoflavanone
Catalog No.:BCN3582
CAS No.:1178893-64-5
- Forsythoside H
Catalog No.:BCN6431
CAS No.:1178974-85-0
- Boc-N-Me-Nle-OH
Catalog No.:BCC2611
CAS No.:117903-25-0
- GLYX 13
Catalog No.:BCC6013
CAS No.:117928-94-6
- Luzindole
Catalog No.:BCC6826
CAS No.:117946-91-5
- Rabeprazole
Catalog No.:BCC5228
CAS No.:117976-89-3
- Rabeprazole sodium
Catalog No.:BCC5227
CAS No.:117976-90-6
- Guanosine
Catalog No.:BCN2962
CAS No.:118-00-3
- Hydrastine
Catalog No.:BCC8187
CAS No.:118-08-1
Compounds exhibiting selective efficacy for different beta subunits of human recombinant gamma-aminobutyric acid A receptors.[Pubmed:15210837]
J Pharmacol Exp Ther. 2004 Nov;311(2):601-9.
Inhibitory GABA(A) receptor modulators are widely used therapeutic agents for a variety of central nervous system disorders. Ltk(-) cells stably expressing human recombinant GABA(A) subunits (alpha1beta1-3gamma2s) were seeded into 96-well plates, loaded with chlorocoumarin-2-dimyristoyl phosphatidylethanolamine and bis(1,3-diethyl-2-thiobarbiturate)trimethineoxonol, and rapid fluorescence resonance energy transfer technique (FRET) measurements were made of GABA-evoked depolarizations in low-Cl(-) buffer using a voltage/ion probe reader. The influence of different betasubunits on the ability of agents to modulate and directly activate the ion channel was examined. GABA evoked concentration-dependent decreases in FRET, increasing fluorescence emission ratio (460/580 nm) at alpha1beta1gamma2, alpha1beta2gamma2, and alpha1beta3gamma2 receptors with similar maximal amplitude (P > 0.05, n = 17) and EC(50) values of 2.4 +/- 0.2, 2.5 +/- 0.2, and 1.3 +/- 0.1 microM, respectively. Piperidine-4-sulfonic acid and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol were less potent, with EC(50) values of 8.7 +/- 0.9, 9.2 +/- 0.5, and 11.7 +/- 1.2, and 43.7 +/- 6.4, 24.8 +/- 1.6, and 26.1 +/- 2.4 microM, respectively. Potency and maximal efficacy of propofol, methyl 6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate, pentobarbital, and steroids, 5alpha-pregnan-3alpha-ol-20-one and 5beta-pregnan-3alpha-ol-20-one, were unaffected by the beta isoform present in the receptor complex. However, several compounds displayed beta2/3 subunit selectivity, notably loreclezole, R(-)-etomidate, and a group of anti-inflammatory agents including mefenamic acid, flufenamic acid, meclofenamic acid, tolfenamic acid, niflumic acid, and diflunisal. The anti-inflammatories exhibited varying levels of efficacy at beta2/3 subunits, with micromolar potency, while having antagonist or weak inverse agonist profiles at alpha1beta1gamma2. Diflunisal was the most efficacious compound, eliciting greater potentiation than loreclezole (90 +/- 14% and 109 +/- 14% at beta3 and beta2, respectively, compared with 62 +/- 6% and 56 +/- 3%), whereas niflumic acid exhibited the lowest efficacy. An additional agent, olsalazine, weakly potentiated responses at all three receptors without any selectivity. This study identifies and characterizes a variety of allosteric modulators for which betasubunits are an important determinant of efficacy and potency.
Loreclezole inhibition of recombinant alpha1beta1gamma2L GABA(A) receptor single channel currents.[Pubmed:10670419]
Neuropharmacology. 2000 Jan 4;39(2):235-45.
Loreclezole had two different effects on GABA(A) receptor (GABAR) currents. When applied to GABARs that contained a beta2 or beta3 subunit subtype, but not a beta1 subtype, loreclezole potentiated the peak current evoked by sub-maximal concentrations of GABA. Loreclezole also increased the rate and degree of apparent desensitization of GABAR whole-cell currents, an effect that was independent of the beta subunit subtype, suggesting that potentiation and inhibition of GABAR current by loreclezole occurred through separate sites. We used patch-clamp recording from outside-out and inside-out patches from L929 fibroblasts transiently transfected with rat GABAR subunits to examine the properties of inhibition of alpha1beta1gamma2L single channel currents by loreclezole. Loreclezole decreased the mean open time of the channel by decreasing the average durations of the open states. Loreclezole also increased the occurrence of a closed component with an average duration near 20 ms. Inhibition by loreclezole was not voltage-dependent. Loreclezole was equally effective when applied to the intracellular side of the receptor, suggesting that its binding site was readily accessible from both sides of the membrane. Pre-application of loreclezole effectively inhibited the GABAR current in macropatches, indicating that binding did not require an open channel. These findings were consistent with a mechanism of allosteric modulation at a site formed by the membrane spanning regions of the receptor.
Loreclezole as a simple functional marker for homomeric rho type GABA(C) receptors.[Pubmed:11080529]
Eur J Pharmacol. 2000 Nov 17;408(2):R1-2.
GABA(C) receptors are expressed in the whole brain, but predominantly in the retina. They can be identified by their unique pharmacology. The establishment of the entire pharmacology is, however, quite tedious. We show here that loreclezole dose dependently inhibits ionic currents elicited by GABA (gamma-aminobutyric acid) with an IC(50) of about 0.5 microM in homomeric rho1 GABA(C) receptors expressed in Xenopus oocytes. Thus, loreclezole may constitute a functional marker for these receptors.
Loreclezole enhances apparent desensitization of recombinant GABAA receptor currents.[Pubmed:9014138]
Neuropharmacology. 1996;35(9-10):1233-41.
Loreclezole is a newly developed antiepileptic drug which has been shown to act at a specific site on beta 2 or beta 3 GABAA receptor subtypes to enhance the peak whole-cell response to submaximal concentrations of GABA. Potentiation by loreclezole occurred with high affinity only at GABAA receptors containing a beta 2 or beta 3 subtype, not a beta 1 subtype. We have studied the effect of loreclezole on whole-cell currents from recombinant GABAA receptors transiently expressed in L929 fibroblasts and on currents from cultured mouse cortical neurons and have found a second, inhibitory action of loreclezole that was independent of the beta-subunit subtype composition of the receptor. Loreclezole, at concentrations above 6 microM, enhanced the degree and rate of apparent desensitization of the whole-cell current in a concentration-dependent manner. This effect was voltage-independent, non-competitive and increased with increasing GABA concentration. The increase in desensitization was not blocked by the benzodiazepine antagonist flumazenil and did not require the presence of a gamma subunit. Loreclezole acted at a novel inhibitory allosteric site to increase the apparent desensitization of the GABAA receptor, regardless of its subunit composition. This activity of loreclezole may have implications for its experimental or clinical use as an antiepileptic drug.


