L-CCG-lPotent group II mGlu agonist CAS# 117857-93-9 |
- Qingyangshengenin A
Catalog No.:BCN8126
CAS No.:106644-33-1
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- Magnoflorine Iodide
Catalog No.:BCN2911
CAS No.:4277-43-4
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117857-93-9 | SDF | Download SDF |
| PubChem ID | 5310956 | Appearance | Powder |
| Formula | C6H9NO4 | M.Wt | 159.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (2<em>S</em>,3<em>S</em>,4<em>S</em>)-CCG | ||
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
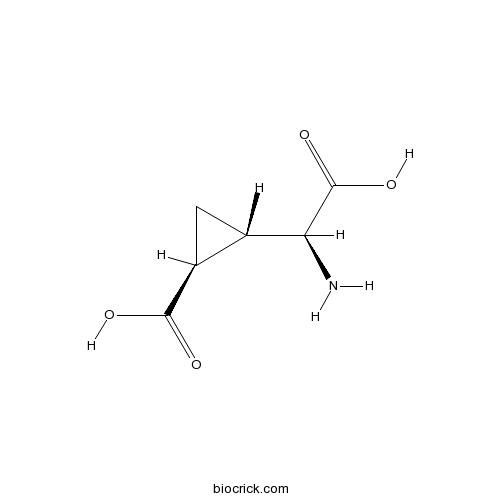
| Chemical Name | (1S,2S)-2-[(S)-amino(carboxy)methyl]cyclopropane-1-carboxylic acid | ||
| SMILES | C1C(C1C(=O)O)C(C(=O)O)N | ||
| Standard InChIKey | GZOVEPYOCJWRFC-HZLVTQRSSA-N | ||
| Standard InChI | InChI=1S/C6H9NO4/c7-4(6(10)11)2-1-3(2)5(8)9/h2-4H,1,7H2,(H,8,9)(H,10,11)/t2-,3-,4-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent group II metabotropic glutamate receptor agonist. More active than glutamate or (±)-trans-ACPD. |

L-CCG-l Dilution Calculator

L-CCG-l Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2838 mL | 31.4189 mL | 62.8378 mL | 125.6755 mL | 157.0944 mL |
| 5 mM | 1.2568 mL | 6.2838 mL | 12.5676 mL | 25.1351 mL | 31.4189 mL |
| 10 mM | 0.6284 mL | 3.1419 mL | 6.2838 mL | 12.5676 mL | 15.7094 mL |
| 50 mM | 0.1257 mL | 0.6284 mL | 1.2568 mL | 2.5135 mL | 3.1419 mL |
| 100 mM | 0.0628 mL | 0.3142 mL | 0.6284 mL | 1.2568 mL | 1.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Loreclezole hydrochloride
Catalog No.:BCC7009
CAS No.:117857-45-1
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- 3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No.:BCN8203
CAS No.:1178-24-1
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- AP-III-a4
Catalog No.:BCC5292
CAS No.:1177827-73-4
- Desmethyl-YM 298198
Catalog No.:BCC7365
CAS No.:1177767-57-5
- Decumbenine C
Catalog No.:BCC8314
CAS No.:117772-89-1
- Azithromycin Dihydrate
Catalog No.:BCC4631
CAS No.:117772-70-0
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- SMANT hydrochloride
Catalog No.:BCC6254
CAS No.:1177600-74-6
- N20C hydrochloride
Catalog No.:BCC7292
CAS No.:1177583-87-7
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
- Fmoc-Thr(Bzl)-OH
Catalog No.:BCC3550
CAS No.:117872-75-0
- 7,4'-Dihydroxyhomoisoflavanone
Catalog No.:BCN3582
CAS No.:1178893-64-5
- Forsythoside H
Catalog No.:BCN6431
CAS No.:1178974-85-0
- Boc-N-Me-Nle-OH
Catalog No.:BCC2611
CAS No.:117903-25-0
- GLYX 13
Catalog No.:BCC6013
CAS No.:117928-94-6
- Luzindole
Catalog No.:BCC6826
CAS No.:117946-91-5
- Rabeprazole
Catalog No.:BCC5228
CAS No.:117976-89-3
- Rabeprazole sodium
Catalog No.:BCC5227
CAS No.:117976-90-6
- Guanosine
Catalog No.:BCN2962
CAS No.:118-00-3
- Hydrastine
Catalog No.:BCC8187
CAS No.:118-08-1
- Cinchonine
Catalog No.:BCN2464
CAS No.:118-10-5
Pharmacology of metabotropic glutamate receptor-mediated enhancement of responses to excitatory and inhibitory amino acids on rat spinal neurones in vivo.[Pubmed:8532150]
Neuropharmacology. 1995 Aug;34(8):1015-23.
Using the technique of microelectrophoresis on spinal neurones in pentobarbitone-anaesthetized rats, (1S,3R)-1-aminocyclo-pentane-1,3-dicarboxylate (1S,3R-ACPD) reversibly and dose-dependently enhanced responses to alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA), kainate, N-methyl-D-aspartate (NMDA) and L-glutamate to a similar extent. 1S,3R-ACPD also enhanced inhibitory responses to both glycine and gamma-aminobutyrate (GABA). Such results are consistent with a metabotropic glutamate receptor-mediated decrease in membrane conductance. 1S,3R-ACPD was the most active metabotropic agonist tested for these effects; the rank order of activity was: 1S,3R-ACPD > or = (2S,3S,4S)alpha-(carboxycyclopropyl)-glycine(L-CCG-l) > (R, S)-3,5-dihydroxy-phenylglycine (3,5-DHPG) > (S)-homoquisqualate > quisqualate = 1S,3S-ACPD > L-2-amino-4-phosphonobutyrate (L-AP4) > 1R,3S-ACPD. These effects of 1S,3R-ACPD were antagonized by (RS)-alpha-methyl-4-carboxy-phenylglycine (M4CPG) and (S)-4-carboxy-3-hydroxy-phenylglycine (4C3HPG) but not by (S)-4-carboxy-phenylglycine (4CPG) or L-amino-3-phosphono-propionate (L-AP3). The pharmacology of the actions of mGluR agonists and antagonists on rat spinal neurones in vivo does not obviously correlate with the published pharmacology of a single cloned metabotropic glutamate receptor subtype but rather suggests that both Group 1 and 2 receptors contribute to the above effects.
Comparative effect of L-CCG-I, DCG-IV and gamma-carboxy-L-glutamate on all cloned metabotropic glutamate receptor subtypes.[Pubmed:9833633]
Neuropharmacology. 1998 Aug;37(8):1043-51.
In a previous study we reported that the addition of a carboxylic group to the mGlu receptor agonist aminocyclopentane-1,3-dicarboxylate (ACPD) changes its properties from agonist to antagonist at both mGlu1 and mGlu2 receptors, and resulted in an increase in affinity at mGlu4 receptors, with isomers being either agonists or antagonists. In the present study, the effect of gamma-carboxy-L-glutamic acid (Gla) and (2S,2'R,3'R)-2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV), two carboxylic derivatives of non-selective agonists, were examined on all cloned mGlu receptors. We found that this additional carboxylic group on glutamate prevents its interaction with group-I mGlu receptors and generates a potent group-II antagonist (K(B) = 55 microM on mGlu2). At group-III mGlu receptors, Gla was found to be either an antagonist (mGlu7 and mGlu8 receptors) or a partial agonist (mGlu4 and mGlu6 receptors). We show here that L-CCG-I is a general mGlu receptor agonist activating all cloned receptors. We also confirm that DCG-IV, which corresponds to L-CCG-I with an additional carboxylic group, is a selective group-II agonist. However, this additional COOH group changes the properties of L-CCG-I from an agonist to an antagonist at all group-III receptors, making this compound one of the most potent group-III mGlu receptor antagonist known so far. These observations will be useful for the development of more potent and selective mGlu receptor agonists and antagonists.
Differentiation of group 2 and group 3 metabotropic glutamate receptor cAMP responses in the rat hippocampus.[Pubmed:8666060]
Eur J Pharmacol. 1996 Feb 22;297(3):275-82.
The effects of group 2- versus group 3-selective metabotropic glutamate (mGlu) receptor agonists were examined against forskolin (10 microM)-, vasoactive intestinal peptide (VIP; 1 microM)- and 5'-N-ethylcarboxamidoadenosine (NECA; 10 microM)-stimulated cAMP accumulations in adult rat hippocampal slices (in the presence of adenosine deaminase). Group 2 mGlu receptor-selective ((1S,3R)-1-aminocyclopentane-1, 3-dicarboxylic acid (1S,3R-ACPD) and (2S,3S,4S)-alpha-(carboxycyclopropyl)-glycine (L-CCG I)) and group 3 mGlu receptor-selective (L-2-amino-4-phosphonobutyric acid (L-AP4) and L-serine-O-phosphate) agonists greatly inhibited forskolin-stimulated cAMP formation ( > 80% at maximally effective concentrations). In contrast, stimulation of cAMP by VIP or NECA was inhibited by group 3, but not by group 2, mGlu receptor agonists. In fact, group 2 mGlu receptor agonists greatly potentiated cAMP accumulation evoked by NECA. Both the inhibitory effects of 1S,3R-ACPD on forskolin-stimulated cAMP and the potentiating effects on NECA-stimulated cAMP accumulation were reversed by the competitive group 1/2 mGlu receptor antagonist (+)-alpha-methyl-4-carboxyphenylglycine ((+)-MCPG). However, (+)-MCPG had no effects on L-AP4 inhibition of cAMP. Thus, the effects of group 2 versus group 3 mGlu receptor agonists on cAMP coupling can be pharmacologically as well as functionally differentiated in the rat hippocampus.
Agonist analysis of 2-(carboxycyclopropyl)glycine isomers for cloned metabotropic glutamate receptor subtypes expressed in Chinese hamster ovary cells.[Pubmed:1330184]
Br J Pharmacol. 1992 Oct;107(2):539-43.
1. 2-(Carboxycyclopropyl)glycines (CCGs) are conformationally restricted glutamate analogues and consist of eight isomers including L- and D-forms. The agonist potencies and selectivities of these compounds for metabotropic glutamate receptors (mGluRs) were studied by examining their effects on the signal transduction of representative mGluR1, mGluR2 and mGluR4 subtypes in Chinese hamster ovary cells expressing the individual cloned receptors. 2. Two extended isomers of L-CCG, L-CCG-I and L-CCG-II, effectively stimulated phosphatidylinositol hydrolysis in mGluR1-expressing cells. The rank order of potencies of these compounds was L-glutamate > L-CCG-I > L-CCG-II. 3. L-CCG-I and L-CCG-II were effective in inhibiting the forskolin-stimulated adenosine 3':5'-cyclic monophosphate (cyclic AMP) accumulation in mGluR2-expressing cells. Particularly, L-CCG-I was a potent agonist for mGluR2 with an EC50 value of 3 x 10(-7) M, which was more than an order of potency greater than that of L-glutamate. 4. L-CCG-I evoked an inhibition of the forskolin-stimulated cyclic AMP production characteristic of mGluR4 with a potency comparable to L-glutamate. 5. In contrast to the above compounds, the other CCG isomers showed no appreciable effects on the signal transduction involved in the three mGluR subtypes. 6. This investigation demonstrates not only the importance of a particular isomeric structure of CCGs in the interaction with the mGluRs but also a clear receptor subtype specificity for the CCG-receptor interaction, and indicates that the CCG isomers would serve as useful agonists for investigation of functions of the mGluR family.
Potent NMDA-like actions and potentiation of glutamate responses by conformational variants of a glutamate analogue in the rat spinal cord.[Pubmed:2692753]
Br J Pharmacol. 1989 Dec;98(4):1213-24.
1. Neuropharmacological actions of all possible-state isomers of alpha-(carboxycyclopropyl)glycine (CCG), conformationally restricted analogues of glutamate, were examined for electrophysiological effects in the isolated spinal cord of the newborn rat. 2. Eight CCG stereoisomers demonstrated a large variety of depolarizing activities. Among them, the (2R, 3S, 4S) isomers of CCG (D-CCG-II) showed the most potent depolarizing activity, followed by the (2S, 3R, 4S) isomer (L-CCG-IV). 3. The depolarization evoked by L-CCG-IV, D-CCG-II and other D-CCG isomers was effectively depressed by N-methyl-D-aspartate (NMDA) antagonists. D-CCG-II was about 5 times more potent than NMDA in causing a depolarization. 4. The (2S, 3S, 4S) isomer of CCG (L-CCG-I) was more potent than L-glutamate in causing a depolarization of spinal motoneurones. The depolarization was slightly depressed by NMDA antagonists, but residual amplitudes of responses to L-CCG-I in the presence of NMDA antagonists We almost insensitive to 6,7-dinitro-quinoxaline-2,3-dione (DNQX) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), suggesting that L-CCG-I might be a novel potent agonist. 5. After application of the (2S, 3S, 4R) isomer of CCG (L-CCG-III), responses to L-glutamate, D- and L-aspartate were markedly enhanced. The enhancement lasted for a period of several hours without a further application of L-CCG-III. 6. L-CCG-III also caused a depolarization, but it seemed unlikely that the potentiation of the glutamate response was directly related to the depolarization evoked by L-CCG-III. 7. The potentiation might be due to inhibition of uptake processes, but L-CCG-III was superior to L-(-)-threo-3-hydroxyaspartate, a potent uptake inhibitor of L-glutamate and L-aspartate, in enhancing the response to L-glutamate in terms of amplitude and duration of responses. 8. CCG isomers should provide useful pharmacological tools for analysis of glutamate neurotransmitter systems.


